Team:Bielefeld-Germany/Labjournal/week6
From 2012.igem.org
(Difference between revisions)
(→Wednesday June 6th) |
(→Thursday June 7th) |
||
| Line 67: | Line 67: | ||
===Thursday June 7th=== | ===Thursday June 7th=== | ||
* '''Team Modeling''': becoming acquainted with matlab while reading the manual | * '''Team Modeling''': becoming acquainted with matlab while reading the manual | ||
| - | * '''Team Bacterial Laccases''': | + | * '''Team Bacterial Laccases''': Colony PCRs of the transformed colonies from yesterday's transformation showed some positive PCR bands. |
| - | + | ||
===Friday June 8th=== | ===Friday June 8th=== | ||
Revision as of 16:27, 22 September 2012
Contents |
Week 6 (06/04 - 06/10/12)
Monday June 4th
- Team A.thaliana Laccase: Primerdesign for isolating a laccase from Arababidopsis thaliana cDNA. Since we want to express the laccase in E.coli we designed the primers like before for the bacterial laccases with T7 promotor and HIS-tag.
- Team Bacterial Laccases:
- The Bacteria S. griseus and S. lavendulae has been delivered so we can start with PCRs. We set the first PCR with them as followed:
- PCR table
- The Bacteria S. griseus and S. lavendulae has been delivered so we can start with PCRs. We set the first PCR with them as followed:
| Material | Volume |
|---|---|
| Buffer (10x Phusion) | 10µL |
| Phusion Polymerase | 0,5µL |
| dNTPs | 1µL |
| Primer Mix | 1µL |
| Template DNA | 1µL |
| DMSO | 1,5µL |
| Water | 35µL |
- PCR program
| Temperature | Time |
|---|---|
| 1) 98°C | 7 mins |
| 2) 98°C | 20 sec |
| 3) 55°C | 20 sec |
| 4) 72°C | 1 min |
| 5) 72°C | 3 min |
| 6) 12°C |
Cycle between step 2 and 4 35 times.
Tuesday June 5th
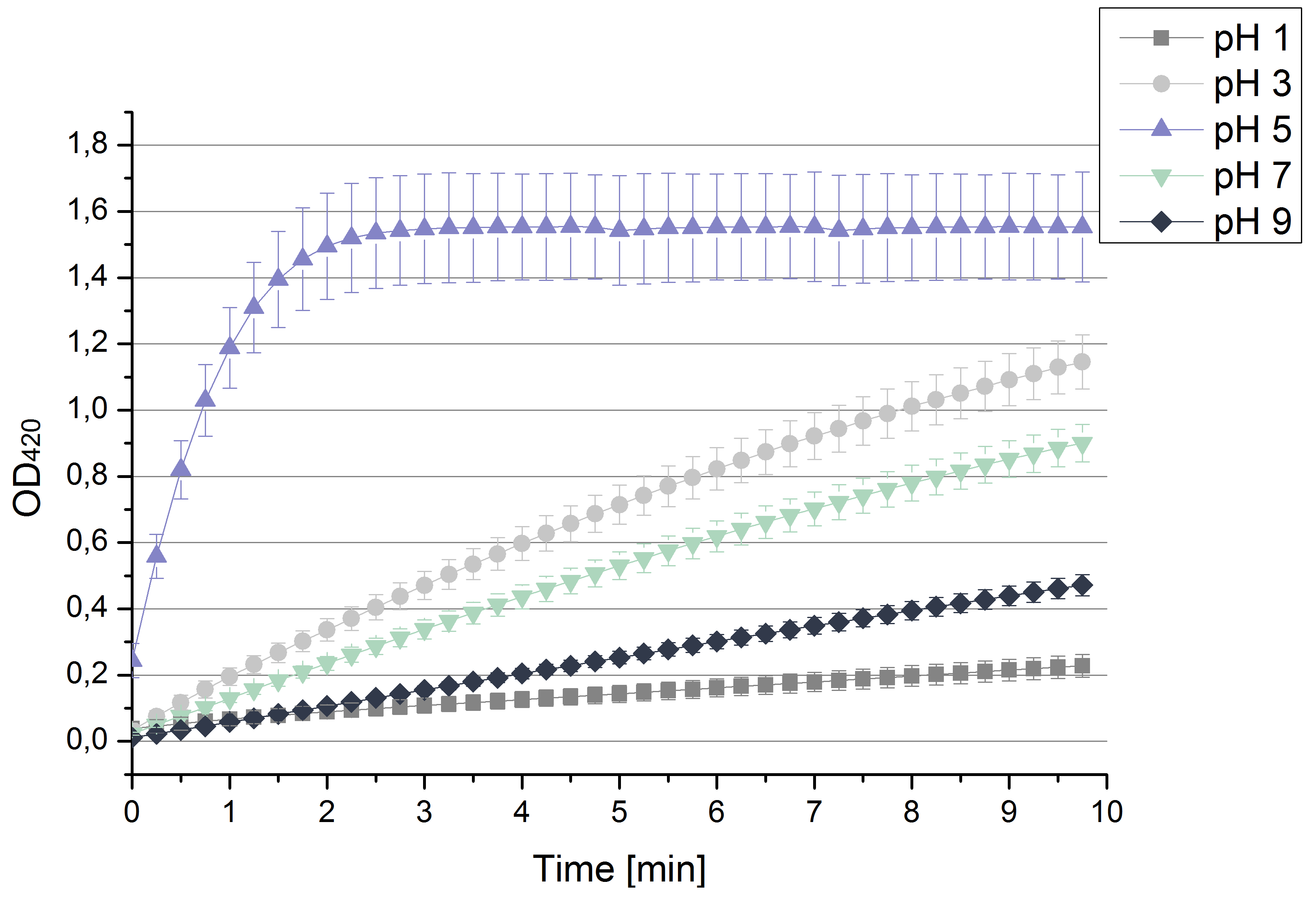
- Team Activity Tests: After testing the T. versicolor laccase under conditions that are optimal (pH 5, 25°C ) according to the literature we now started further characterization under different pHs. We analyzed the laccases behavior when working in 100 mM sodium acetate buffer at pH 1, 3, 7 an 9. Result: We agree with the literature that pH 5 seems to make the laccase happy. Since not all waste waters (especially those here in Germany) are not as warm as 25°C we now wonder what our laccase might do when exposed to lower temperaturers. Stay tuned.
- Team Bacterial Laccases:
- The sequencing results for isolated plasmids xccl(T7)_His, bpul(T7)_His and ecol(T7)_His came. The results showed that only the xccl(T7)_His was ok – our first finished biobrick *yeha*. We proudly name it <partinfo>BBa_K863015</partinfo>. The sequence of 'ecol(T7)_His showed that there are missing 4 bases in the promoter region and the bpul(T7)_His sequence showed a mutation which leads to another amino acid in protein sequence.
- Again we did PCRs on T. thermophilus laccase and B. halodurans laccase with B.halo_FW_T7 / B.halo_FW_HIS and T.thermo_LAC_FW_T7 / T.thermo_LAC_RV_HIS primers and purified the product,this time with enough material for a restriction.
Wednesday June 6th
- Team Wiki: Yay for Team Wiki´s first entry. Our first steps with the iGEM Bielefeld 2012 Wiki contain thinking about contents, layouts, programming and responsibilities. Our first rules are:
- we are programming static pages in HTML and all the other pages (those that will be updated by all team members) in wiki code.
- we created all pages and will fill them up with some nice and beautiful content constantly from now on.
- Our temporary banner contains our outstanding logo and a DNA but we will set up a new layout soon.
- Team Bacterial Laccases: Digest of tthl(T7)_His and bhal(T7)_His PCR products and ligation in pSB1C3 backbone. After that we transformed the plasmids in competent E. coli KRX cells.
Thursday June 7th
- Team Modeling: becoming acquainted with matlab while reading the manual
- Team Bacterial Laccases: Colony PCRs of the transformed colonies from yesterday's transformation showed some positive PCR bands.
Friday June 8th
Team Bacterial Laccases:
- We plated colonies for plasmid isolations on new plates and made a control restriction with NotI. The electrophoretic separation showed gel bands in the right hight for the Tth-plasmid and the plasmid with B. halodurans laccase.
Saturday June 9th
Sunday June 10th
| 55px | | | | | | | | | | |
 "
"