Team:Bielefeld-Germany/Labjournal/week13
From 2012.igem.org
(Difference between revisions)
(→Saturday July 28th) |
(→Monday July 23rd) |
||
| Line 14: | Line 14: | ||
** Was the laccase produced but not in a high enough amount so that we can´t detect its activity in the first place? | ** Was the laccase produced but not in a high enough amount so that we can´t detect its activity in the first place? | ||
Another thought was that there might be copper missing. Since laccase is a copper-enzyme it might be necessary to add copper to the medium whenever it is produced in such high amounts. It´s late, so we will do that tomorrow. | Another thought was that there might be copper missing. Since laccase is a copper-enzyme it might be necessary to add copper to the medium whenever it is produced in such high amounts. It´s late, so we will do that tomorrow. | ||
| - | * '''Team Cloning of Bacterial | + | * '''Team Cloning of Bacterial Laccases''': The transformation from the 21st July showed no results. |
| - | + | ||
* '''Team Fungal and Plant Laccases:''' Successful PCRs of laccase gene Tv5 from ''Trametes versicolor'' with plasmid DNA from Uni Greifswald as template. | * '''Team Fungal and Plant Laccases:''' Successful PCRs of laccase gene Tv5 from ''Trametes versicolor'' with plasmid DNA from Uni Greifswald as template. | ||
* '''Team Site Directed Mutagenesis:''' Transfered 4 colonys of each transformation to an new dish for plasmid-isolation | * '''Team Site Directed Mutagenesis:''' Transfered 4 colonys of each transformation to an new dish for plasmid-isolation | ||
Revision as of 18:31, 22 September 2012
Contents |
Week 13 (07/23 - 07/29/12)
- From 07/23 - 07/25/12, some of our team members participated at the CAS conference in Munich.
Monday July 23rd
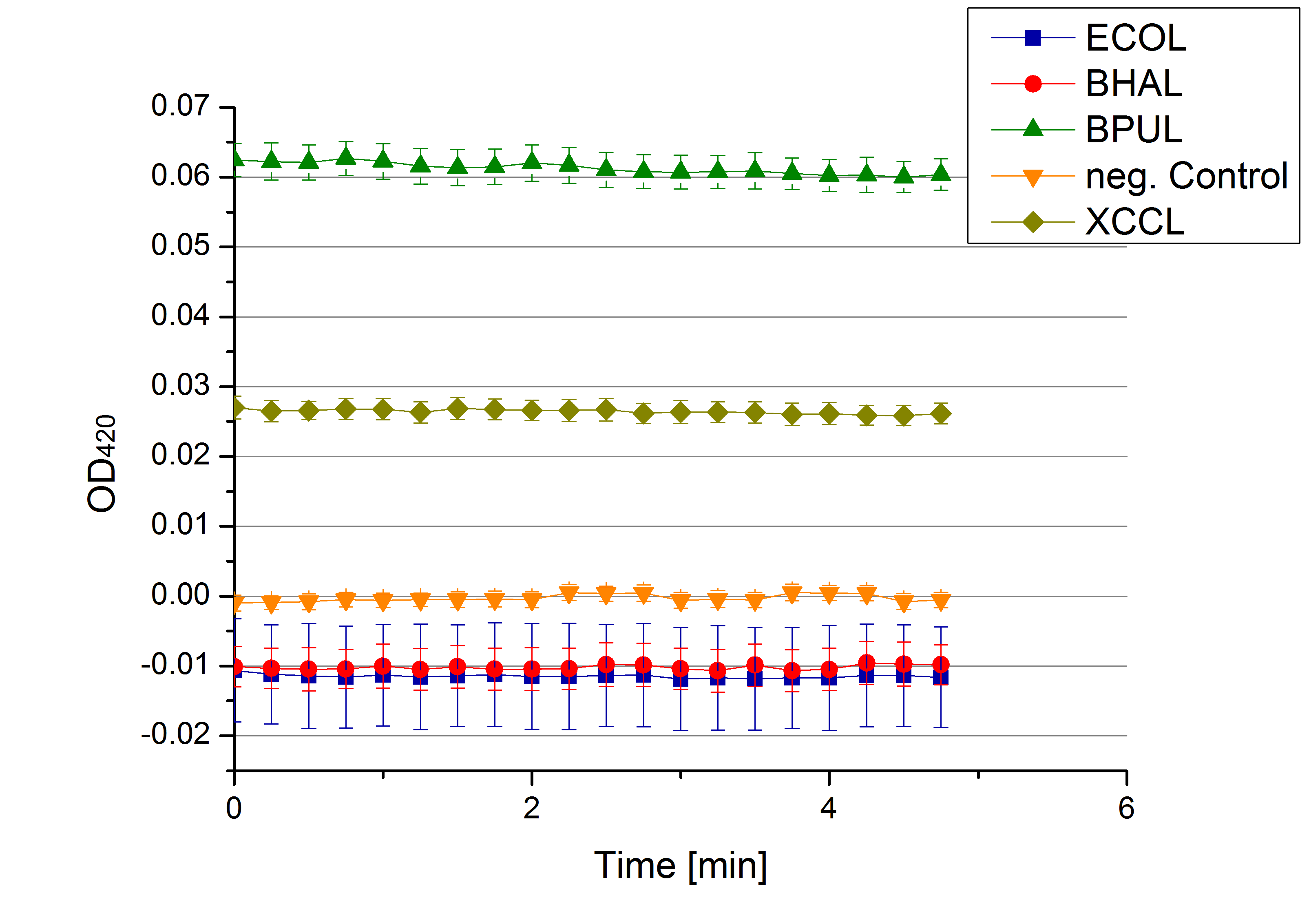
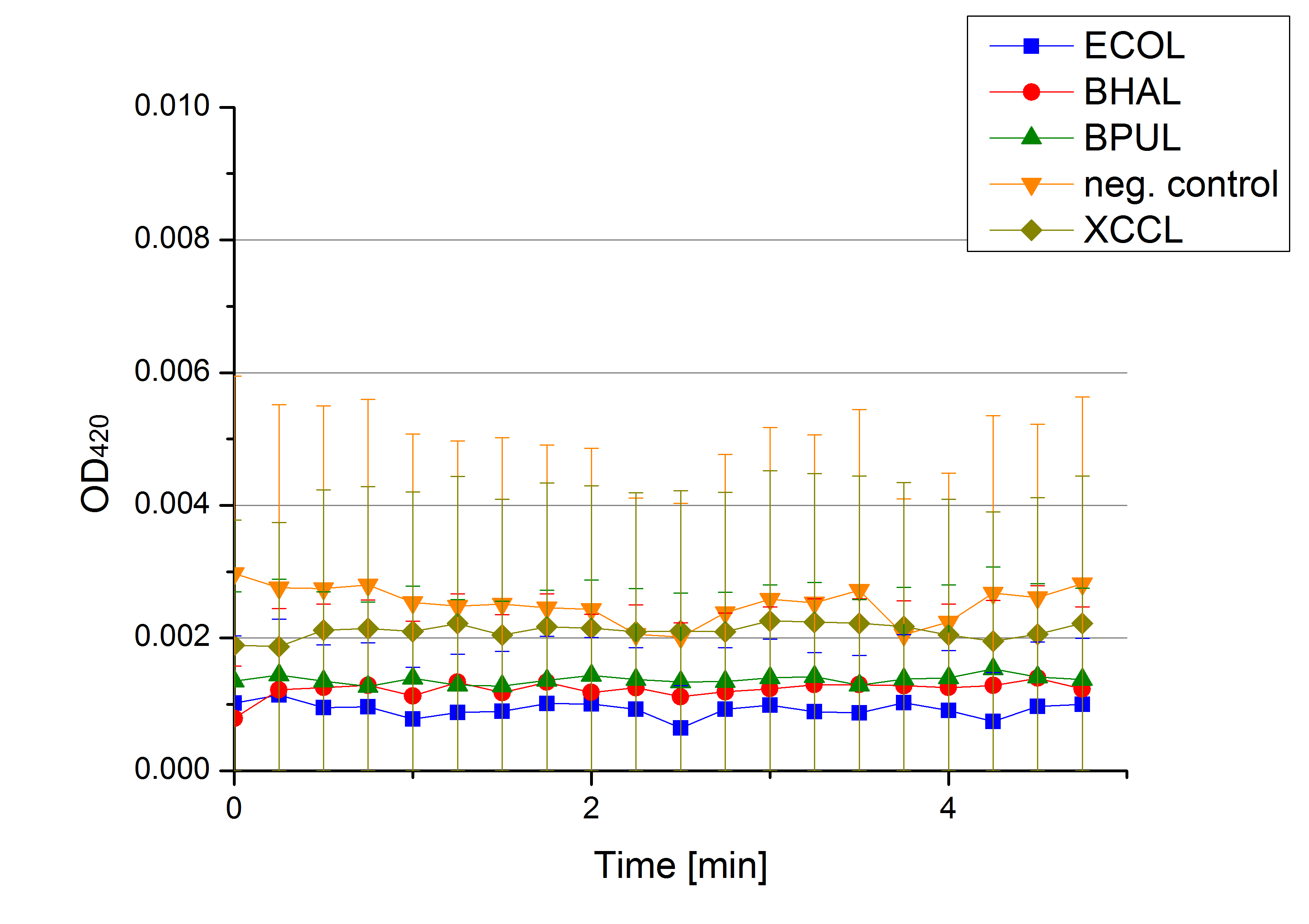
- Team Activity Tests: Today was the moment of truth. We received the first recombinant laccases from the cultivation team. We started with testing the supernatant from the cultivated cells to check whether they secrete laccase. We added 0,1 mM ABTS in each well that was filled with supernatant from E.coli KRX that contained the plasmid to produce ECOL, BPUL, BHAL, XCCL or E. coli KRX (negative control) and measured as usual. There was no change in the OD so that we assume that no secretion takes place. Afterwards we tested potential laccase activity by filling each well with laccase and buffer and added 0,1 mM ABTS. Unfortunately no activity was seen. We felt like we could not take the laziness of our laccases. We thought and discussed what the reason could be for them to be inactive.
- Is there something wrong with the transformed construct, maybe a mutation?
- Was the laccase synthesized but is inactive for some reason?
- Was the laccase produced but not in a high enough amount so that we can´t detect its activity in the first place?
Another thought was that there might be copper missing. Since laccase is a copper-enzyme it might be necessary to add copper to the medium whenever it is produced in such high amounts. It´s late, so we will do that tomorrow.
- Team Cloning of Bacterial Laccases: The transformation from the 21st July showed no results.
- Team Fungal and Plant Laccases: Successful PCRs of laccase gene Tv5 from Trametes versicolor with plasmid DNA from Uni Greifswald as template.
- Team Site Directed Mutagenesis: Transfered 4 colonys of each transformation to an new dish for plasmid-isolation
Tuesday July 24th
- Team Site Directed Mutagenesis: Plasmid-isolation of all 16 different colony-dishes
- Team Activity Test: Today is copper day! First we divided each sample (those from yesterday) and added 2mM or 4mM to the halfs, respectively. We incubated our samples for 2h and hoped that the concentration gradient would cause the laccases to exchange the ion they have integrated instead against copper. We measured their activity again after those 2h but there was no change seen. Team Cultivation plans to add copper from the very beginning of cultivation next time.
- Team Fungal and Plant Laccase: Since our cDNA is now ready to go and also our primers already arrived we started a PCR. Check protocols for more information about the exact setup. Unfortunately no bands could be seen via gel electrophoresis. We blamed the high salt concentration and decided that a some cleaning via ethanol precipitation might be a solution to our problem.
Wednesday July 25th
- Team Site Directed Mutagenesis: Test-digestion (with the enzym of the mutated restriction-site) of the Plasmids from the 16 different colonies revealed that two bpul, one ecol and two tthl mutants have the right bands in the gel and seem be mutated correctly. Xccl-colonies are all unmutated (no Band at 3636).
- pfu-PCRs with the two correct bpul-plasmids as template and the bpul-g2317t primer-mix
- two new pfu-PCRs with the xccl-plasmid one with the g2247c primer-mix and the other with the xccl-g3633c primer-mix
- Prepared all positive colones for sequencing
- Team Cultivation & Purification:
- We made precultures of E. coli KRX without plasmid and with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863005 BBa_K863005] , pBpL6 as well as with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863020 BBa_K863020] or [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863015 BBa_K863015].
Thursday July 26th
- Team Modeling and Team Sponsoring: meeting Mr. ??? from the clarification plant of Schloß Holte and finding a new cooperation partner. He want to give us the information we need to equate our model and design our cleaner. On top he wants to ask if the clarification plant can sponsor our project.
- Team Site Directed Mutagenesis: Went on with the PCR products of xccl and bpul, transformed them into XL1 Blue and plated them on select-agar
- pfu-PCRs with the tvel10-plasmid, one with the tvel10-243 primer-mix and one with the tvel10-1161 primer-mix. Both PCRs showed correct bands for the PCR-product in a test-agarose-gel-electrophoresis. Digested template with DpnI.
- Team Fungal and Plant Laccases: We did PCR on Trametes versicolor laccase tvel5 and Pycnoporus cinnabarinus pcil35 with Tv_lac10.P.FW / Tv_lac10.S.RV and Pc_lac35.P.FW / Pc_lac35.S.RV primers. Additionally we want the laccases with overhanging ends for cloning in our shuttle vector for expression in Pichia pastoris. Therefore we used Pc_lac35_FW_oS / Pc_lac35_RV and Tv_lac5_FW_oS / Tv_lac5_RV primer pairs.
- Team Cultivation & Purification:
- Glycerine cultures were made of all given BioBricks, to use them for precultures.
- Made new precultures analogous to 07/25.
- Found out, that it could be important to add CuCl2 to the medium, because the copper is important for the active center of the laccases and to improve their stability. As an alternative we could incubate them with copper before measuring the activity.
Friday July 27th
- Team Site Directed Mutagenesis: plated four colonies of each transformation-dish (xccl & bpul) on a petri dish for plasmid-isolation. Purified the digested PCR-products of tvel10.
- Team Cellulose Binding Domain:
- We made the decision to only use cellolose binding domains (CBDs) and not carbohydrate binding domains of any kind to keep them comparable. This means we won't use the binding domains of Bacillus halodurans.
- Redesigned Primers for [http://partsregistry.org/Part:BBa_K863112 CBDclos] - added the bases TA in between RBS and ATG since the RBS can be made stronger by adding more adenines in the sequence upstream of the RBS.
- Made similar primers for [http://partsregistry.org/Part:BBa_K863102 CBDcex] (the CBD of the Cellulomonas fimi exoglucanase we got for the fermentation-group of Bielefeld University)
- Checked the Wiki of the iGEM 2012 Osaka University-Team about their <partinfo>BBa_K392014</partinfo> BioBrick which should be a binding motif, but is the glycosyl hydrolase domain, for their intentions. We guess they really messed it up. It would have been nice to have a CBD from the partsregistry.
- Team Fungal and Plant Laccases: The PCR on tvel5 laccase was positive after the first trial for both primer pairs. For the tvel35 laccase the PCR didn’t work.
- Team Cultivation & Purification:
- We cultivated E. coli KRX with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863005 BBa_K863005], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863020 BBa_K863020], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863015 BBa_K863015] as well as pBpL6. As negative control we used E. coli KRX.
- Settings: 300 mL flasks without baffles, final volume: 60 mL, autoinduction medium, 0,25 mM CuCl2, 30 °C
- We made glycerine cultures of homologous culture of E. coli KRX with pBpL6.
- We cultivated E. coli KRX with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863005 BBa_K863005], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863020 BBa_K863020], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863015 BBa_K863015] as well as pBpL6. As negative control we used E. coli KRX.
Saturday July 28th
- Team Activity Tests: See, we are back already! Today we received different samples from Escherichia coli, Bacillus pumilus, Bacillus halodurans C-125, Thermus thermophilus, Xanthomonas campestris pv. campestris B100 and E. coli KRX (negative control - cells without plasmid). The cells were all cultivated at 30°C and supplied with copper. We tested each one of them but none of them showed laccase activity.
- Team Fungal and Plant Laccases: Purification of Trametes versicolor tvel5 PCR pruduct.
- Team Cultivation & Purification:
- Cells of cultivation 07/27 were disrupted via sonification and given to the activity test team.
Sunday July 29th
- Team Cultivation & Purification:
- Preparation of all the stuff needed for SDS-Pages.
- Made a new preculture of E. coli KRX without plasmid and with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863005 BBa_K863005], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863020 BBa_K863020], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863015 BBa_K863015], as well as pBpL6 and our new BioBricks [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863005 BBa_K863005] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863010 BBa_K863010].
| 55px | | | | | | | | | | |
 "
"