Team:Bielefeld-Germany/Results/thermo
From 2012.igem.org
(→Since Regionals: Purification of TTHL since Regionals) |
(→Substrate Analysis) |
||
| (69 intermediate revisions not shown) | |||
| Line 13: | Line 13: | ||
<p class="more"> | <p class="more"> | ||
</html> | </html> | ||
| - | Initially some trials of shaking flask cultivations were made with different parameters to identify the best conditions for the production of the His-tagged laccase LttH from [http://www.dsmz.de/catalogues/details/culture/DSM-7039.html?tx_dsmzreso ''Thermus thermophilus'' HB27] named TTHL. Due to the absence of enzyme activity of the enzyme in the cell lysate a purification method was established (using Ni-NTA-His tag resin). Using ''E. coli'' KRX containing BioBrick <partinfo>BBa_K863010</partinfo>, TTHL could not be detected by SDS-PAGE (molecular weight of 53 kDa) or by activity test. Therefore a new BioBrick <partinfo>BBa_K863012</partinfo> was constructed and expressed in ''E. coli'' Rosetta-Gami 2. With this expression system the TTHL could be detected by SDS-PAGE and purified by using a small scale Ni-NTA column. The fractionated samples were tested regarding their activity. TTHL was shown to oxidize ABTS. After measuring activity of TTHL a scale up of the fermentation was successfully implemented up to 6 L. A further scale up to 12 L with a optimized medium (HSG) and a labscale Ni-NTA-Purification were implemented to enable additional experiments to characterize TTHL. | + | Initially some trials of shaking flask cultivations were made with different parameters to identify the best conditions for the production of the His-tagged laccase LttH from [http://www.dsmz.de/catalogues/details/culture/DSM-7039.html?tx_dsmzreso ''Thermus thermophilus'' HB27] named TTHL. Due to the absence of enzyme activity of the enzyme in the cell lysate a purification method was established (using Ni-NTA-His tag resin). Using ''E. coli'' KRX containing BioBrick <partinfo>BBa_K863010</partinfo>, TTHL could not be detected by SDS-PAGE (molecular weight of 53 kDa) or by activity test. Therefore a new BioBrick <partinfo>BBa_K863012</partinfo> was constructed and expressed in ''E. coli'' Rosetta-Gami 2. With this expression system the TTHL could be detected by SDS-PAGE and purified by using a small scale Ni-NTA column. The fractionated samples were tested regarding their activity. TTHL was shown to oxidize ABTS. After measuring activity of TTHL a scale up of the fermentation was successfully implemented up to 6 L. A further scale up to 12 L with a optimized medium (HSG) and a labscale Ni-NTA-Purification were implemented to enable additional experiments to characterize TTHL. A total specific enzyme activity of 15 U mg<sup>-1</sup> was determined for TTHL at pH 4 at 25°C with ABTS as a substrate. |
| Line 65: | Line 65: | ||
[[File:Bielefeld2012_TTHL12L.jpg|450px|thumb|left|'''Figure 3:''' Fermentation of ''E. coli'' Rosetta-Gami 2 with <partinfo>BBa_K863012</partinfo> (TTHL) in a Bioengineering NFL22. Conditions: 12 L of [https://2012.igem.org/Team:Bielefeld-Germany/Protocols/Materials#HSG_Autoinduction_medium HSG autoinduction medium] + 60 µg/mL chloramphenicol at 37 °C, pH 7. Agitation increased when pO<sub>2</sub> was below 50 % and OD<sub>600</sub> was measured each hour.]] | [[File:Bielefeld2012_TTHL12L.jpg|450px|thumb|left|'''Figure 3:''' Fermentation of ''E. coli'' Rosetta-Gami 2 with <partinfo>BBa_K863012</partinfo> (TTHL) in a Bioengineering NFL22. Conditions: 12 L of [https://2012.igem.org/Team:Bielefeld-Germany/Protocols/Materials#HSG_Autoinduction_medium HSG autoinduction medium] + 60 µg/mL chloramphenicol at 37 °C, pH 7. Agitation increased when pO<sub>2</sub> was below 50 % and OD<sub>600</sub> was measured each hour.]] | ||
<div style="text-align:justify;"> | <div style="text-align:justify;"> | ||
| - | Another scale-up of the fermentation of ''E. coli'' Rosetta-Gami 2 with <partinfo>BBa_K863012</partinfo> was made up to a final working volume of 12 L in a Bioengineering NFL 22 fermenter. Agitation speed, pO<sub>2</sub> and OD<sub>600</sub> were determined | + | Another scale-up of the fermentation of ''E. coli'' Rosetta-Gami 2 with <partinfo>BBa_K863012</partinfo> was made up to a final working volume of 12 L in a Bioengineering NFL 22 fermenter. Agitation speed, pO<sub>2</sub> and OD<sub>600</sub> were determined as well as the glycerin concentration. The data are illustrated in Figure 3. This time [https://2012.igem.org/Team:Bielefeld-Germany/Protocols/Materials#HSG_Autoinduction_medium HSG autodinduction medium] was used to produce more biomass. Due to the change of media and to a low amount of cells for inocculation, there was a long lag phase of about 6 hours. During this phase the glycerin concentration is nearly constant. The cells were in an exponential phase between 8 and 18 hours of cultivation, which results in a decrease of gylcerin, of pO<sub>2</sub> value and therefore in an increase of agitation speed. After 18 hours of cultivation the maximal OD<sub>600</sub> of 9.63 was reached and the glycerin was completely consumed. At that time the cells were just entering the stationary phase. No further data for OD<sub>600</sub> were measured. The cells have been harvested after 22 hours of cultivation. In the review, to leave the cells longer in the stationary phase could have been a better procedure concerning the yield. |
<br style="clear: both" /> | <br style="clear: both" /> | ||
| Line 73: | Line 73: | ||
<p align="justify"> | <p align="justify"> | ||
| - | The harvested cells were resuspended in [https://2012.igem.org/wiki/index.php?title=Team:Bielefeld-Germany/Protocols/Materials#Buffers_for_His-Tag_affinity_chromatography Ni-NTA- equilibration buffer] and mechanically disrupted by [https://2012.igem.org/Team:Bielefeld-Germany/Protocols/Production#Mechanical_lysis_of_the_.28bio-reactor.29_cultivation homogenization]. The cell debris were removed by centrifugation and microfiltration via [http://www.millipore.com/catalogue/module/C7493 Millipore Pellicon XL 50]. The supernatant of the cell lysate was concentrated with [http://www.millipore.com/catalogue/module/C7493 Millipore Pellicon XL 50] with 10 kDa and loaded on the Ni-NTA column (15 mL Ni-NTA resin) with a flow rate of 1 mL min<sup>-1</sup> cm<sup>-2</sup>. Then the column was washed with 10 column volumes (CV) [https://2012.igem.org/wiki/index.php?title=Team:Bielefeld-Germany/Protocols/Materials#Buffers_for_His-Tag_affinity_chromatography Ni-NTA equilibration buffer]. The bound proteins were eluted by an increasing [https://2012.igem.org/wiki/index.php?title=Team:Bielefeld-Germany/Protocols/Materials#Buffers_for_His-Tag_affinity_chromatography Ni-NTA elution buffer] step elution from 5 % (equates to 25 mM | + | The harvested cells were resuspended in [https://2012.igem.org/wiki/index.php?title=Team:Bielefeld-Germany/Protocols/Materials#Buffers_for_His-Tag_affinity_chromatography Ni-NTA- equilibration buffer] and mechanically disrupted by [https://2012.igem.org/Team:Bielefeld-Germany/Protocols/Production#Mechanical_lysis_of_the_.28bio-reactor.29_cultivation homogenization]. The cell debris were removed by centrifugation and microfiltration via [http://www.millipore.com/catalogue/module/C7493 Millipore Pellicon XL 50]. The supernatant of the cell lysate was concentrated with [http://www.millipore.com/catalogue/module/C7493 Millipore Pellicon XL 50] with 10 kDa and loaded on the Ni-NTA column (15 mL Ni-NTA resin) with a flow rate of 1 mL min<sup>-1</sup> cm<sup>-2</sup>. Then the column was washed with 10 column volumes (CV) [https://2012.igem.org/wiki/index.php?title=Team:Bielefeld-Germany/Protocols/Materials#Buffers_for_His-Tag_affinity_chromatography Ni-NTA equilibration buffer]. The bound proteins were eluted by an increasing [https://2012.igem.org/wiki/index.php?title=Team:Bielefeld-Germany/Protocols/Materials#Buffers_for_His-Tag_affinity_chromatography Ni-NTA elution buffer] step elution from 5 % (equates to 25 mM imidazole) with a length of 50 mL, to 50 % (equates to 250 mM imidazole) with a length of 80 mL and finally to 100 % (equates to 500 mM imidazole) with a length of 80 mL. This strategy was chosen to improve the purification caused by a step by step increasing Ni-NTA-elution buffer concentration. The elution was collected in 10 mL fractions. In Figure 3 only the UV-detection signal of the wash step and the elution are shown, this is because of the high UV-detection signal of the loaded samples and to simplify the illustration of the detected product peak. A typical chromatogram of purified laccases is illustrated [https://static.igem.org/mediawiki/2012/4/49/Bielefeld2012_Chromatogram_examplegrafik.jpg here]. The chromatogram of the TTHL elution is shown in Figure 4: |
</p> | </p> | ||
| - | [[File:Bielefeld2012 TTHL Chromatogramm.jpg|450px|thumb|left|'''Figure | + | [[File:Bielefeld2012 TTHL Chromatogramm.jpg|450px|thumb|left|'''Figure 4:''' Chromatogram of wash and elution fractions from FLPC Ni-NTA His-tag Purification of TTHL produced by 12 L fermentation of ''E. coli'' Rosetta Gami 2 with <partinfo>BBa_K863012</partinfo>. TTHL was eluted by a concentration of 50 % (equates to 250 mM imidazole) with a maximal UV-detection signal of 123 mAU. ]] |
<p align="justify"> | <p align="justify"> | ||
| - | Contrary to our expectations, the chromatogram shows one distinguished peak. This peak was detected at a [https://2012.igem.org/wiki/index.php?title=Team:Bielefeld-Germany/Protocols/Materials#Buffers_for_His-Tag_affinity_chromatography Ni-NTA-equilibration buffer] concentration of 100 % (equates to 500 mM | + | Contrary to our expectations, the chromatogram shows one distinguished peak. This peak was detected at a [https://2012.igem.org/wiki/index.php?title=Team:Bielefeld-Germany/Protocols/Materials#Buffers_for_His-Tag_affinity_chromatography Ni-NTA-equilibration buffer] concentration of 100 % (equates to 500 mM imidazole) and resulted from the elution of bound protein. Earlier measurements of other bacterial laccases showed that the elution of these laccases begins with a elution buffer concentration of 50 % equates to 250 mM imidazole. One explanation of this result could be a low concentration of the produced TTHL. Consequently all elution fractions were analyzed by SDS-PAGE to detect TTHL. In the chromatogram no further peaks were detected. The following increasing UV detection signal by increasing concentration of the elution buffer results from the rising imidazole concentration of the Ni-NTA elution buffer. The corresponding SDS-PAGES are shown in Figure 5. |
</p> | </p> | ||
| + | <br style="clear: both" /> | ||
| + | |||
| + | ===Since Regionals: SDS-PAGE of protein purification=== | ||
| + | [[File:Bielefeld2012_1019thermo.jpg|300px|thumb|left|'''Figure 5:''' SDS-PAGE of purification from the 12 L fermentations from 10/11 ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 BBa_K863012]). Purification of the supernatant via microfiltration, diafiltration and Ni-NTA column (step gradient with 5 %, 50 % and 100 % elution buffer).]] | ||
| + | |||
| + | In Figure 5 the SDS-PAGE of the Ni-NTA purification of the lysed ''E.coli'' Rosetta-Gami 2 culture containing [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 BBa_K863012] is illustrated. It shows the permeate and retentate of microfiltration and diafiltration respectively, several fractions of flow-through, wash and the elutions with different buffer concentrations respectively. The selected samples were taken where peaks were seen in the chromatogram. The His-tagged TTHL has a molecular weight of 53 kDa. Apparently the concentration of TTHL is too low to see a band. | ||
| + | |||
| + | |||
<br style="clear: both" /> | <br style="clear: both" /> | ||
| Line 87: | Line 95: | ||
===Initial activity tests of purified fractions=== | ===Initial activity tests of purified fractions=== | ||
<p align="justify"> | <p align="justify"> | ||
| - | There was no activity measurable after cultivation and purification of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863022 BBa_K863022] under the control of a T7 promoter. Activity tests of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL] under a constitutive promoter did reveal TTHL laccases capable of oxidizing ABTS. Fractions 1 to 5 of the purification above were rebuffered with deionized H<sub>2</sub>O and incubated with 0.4 mM CuCl<sub>2</sub> for 2 hours. Activity measurements were performed using 140 µL sample, 0.1 mM ABTS and 100 mM sodium acetate buffer (pH 5) to a final volume of 200 µL. The change in optical density at 420 nm was detected, reporting the oxidization of ABTS through laccases. Fractions | + | There was no activity measurable after cultivation and purification of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863022 BBa_K863022] under the control of a T7 promoter. Activity tests of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL] under a constitutive promoter did reveal TTHL laccases capable of oxidizing ABTS. Fractions 1 to 5 of the purification above were rebuffered with deionized H<sub>2</sub>O and incubated with 0.4 mM CuCl<sub>2</sub> for 2 hours. Activity measurements were performed using 140 µL sample, 0.1 mM ABTS and 100 mM sodium acetate buffer (pH 5) to a final volume of 200 µL. The change in optical density at 420 nm was detected, reporting the oxidization of ABTS through laccases. Fractions 1 to 5 show activity (Figure 6). Fraction 2 seems to contain most of TTHL showing the highest activity compared to the other fractions: 40 % of the used ABTS has been oxidized after 2 hours. Based on these results protein concentrations have to be determined and the activity of the TTHL laccase can be characterized in further experiments including pH optimum and activity in regard of temperature shifts. |
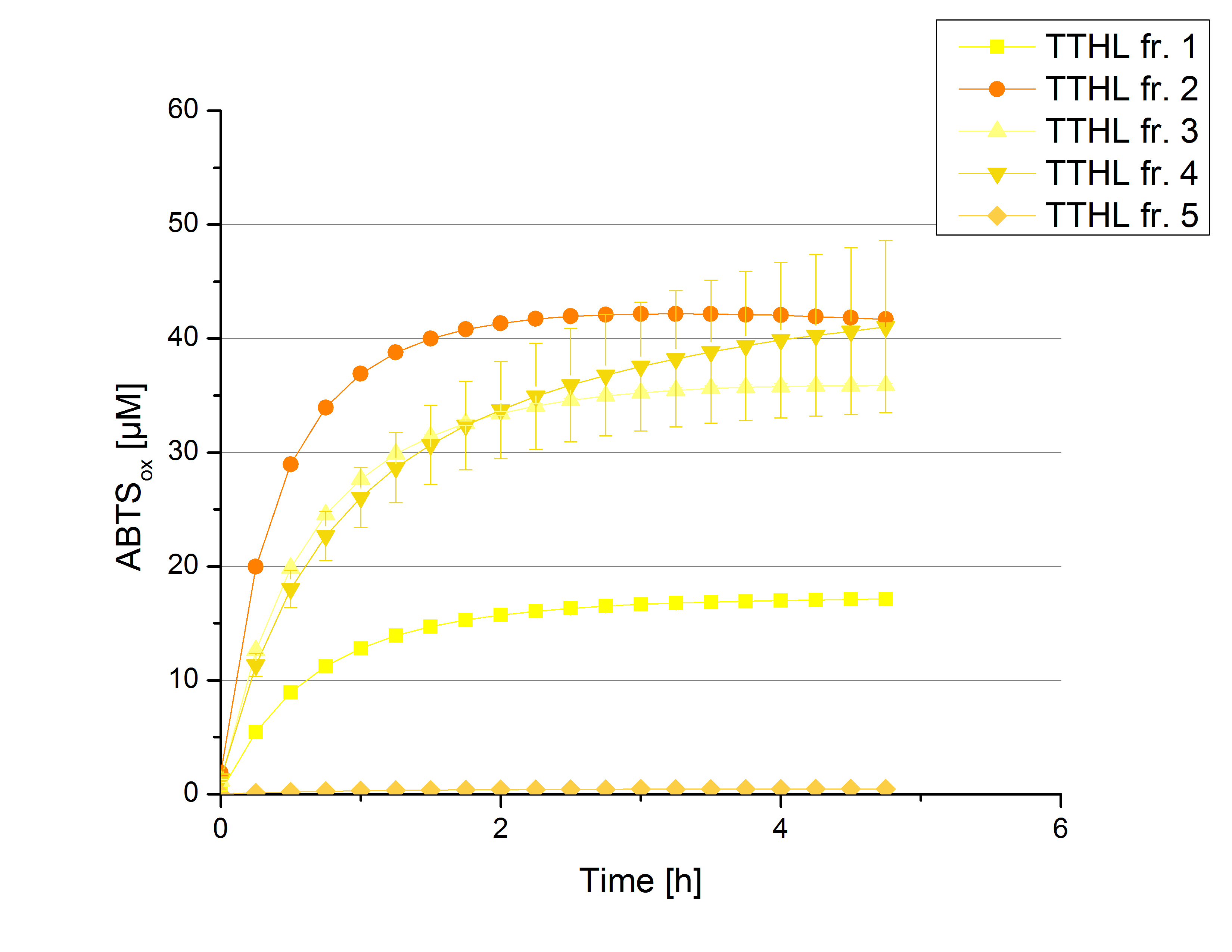
| - | [[File:Bielefeld2012_17_09_TTHL1.jpg|thumbnail|450px|center|'''Figure | + | [[File:Bielefeld2012_17_09_TTHL1.jpg|thumbnail|450px|center|'''Figure 6:''' Activity test of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL] fractions resulting from the purification. Reaction setup includes 140 µL fraction sample (CuCl<sub>2</sub> incubated), 0.1 mM ABTS and 100 mM sodium actetate buffer (pH 5) to a final volume of 200 µL. Measurements were done at 25 °C and over a time period of 5 hours. [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL] shows activity in oxidizing ABTS except fractions 1 seems to have no active [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL]. (n=4)]] |
<br style="clear: both" /> | <br style="clear: both" /> | ||
| - | |||
===Since Regionals: Initial activity tests of purified fractions=== | ===Since Regionals: Initial activity tests of purified fractions=== | ||
| - | The purificated fractions of the cultivation after the Regional Jamborees in Amsterdam were tested concerning their [https://2012.igem.org/Team:Bielefeld-Germany/Amsterdam/Labjournal#Tuesday_October_16th/ protein concentration]. After re-buffering the [https://2012.igem.org/Team:Bielefeld-Germany/Amsterdam/Labjournal#Tuesday_October_17th protein concentration] was determined again and all fraction were incubated with 0.4 mM CuCl<sub>2</sub>. For the initial activity test the protein amount was adjusted for comparison. The fractions were measured in Britton-Robinson buffer at pH 5 with 0.1 mM ABTS. Fraction 50% 1 showed the highest activity (Fig. | + | The purificated fractions of the cultivation after the Regional Jamborees in Amsterdam were tested concerning their [https://2012.igem.org/Team:Bielefeld-Germany/Amsterdam/Labjournal#Tuesday_October_16th/ protein concentration]. After re-buffering the [https://2012.igem.org/Team:Bielefeld-Germany/Amsterdam/Labjournal#Tuesday_October_17th protein concentration] was determined again and all fraction were incubated with 0.4 mM CuCl<sub>2</sub>. For the initial activity test the protein amount was adjusted for comparison. The fractions were measured in Britton-Robinson buffer at pH 5 with 0.1 mM ABTS. Fraction 50 % 1 showed the highest activity (Fig. 7). Regarding the protein amount of this fraction and the statement, that 90 % of this are TTHL laccase, fraction 50 % 1 contains 4,03 µg mL<sup>-1</sup>. To ensure enough protein for further experiments, the second best fraction, which is fraction 5 % 3 was added to fraction 50 % 1. The first number indicates the percentage of used elution buffer, whereas the second number stands for the fraction number of this elution. In total, both fraction contain [https://2012.igem.org/Team:Bielefeld-Germany/Amsterdam/Labjournal#Tuesday_October_19th/ 4,4 µg mL<sup>-1</sup>]. |
| - | [[File:Bielefeld2012_new_TTHL_activity.jpg|500px|thumb|center|Activity assay of each purified fraction of the new cultivation with TTHL. Samples were re-buffered into H<sub>2</sub>O and the protein amount in each fraction has been adjusted. The measurements were done using the [https://2012.igem.org/Team:Bielefeld-Germany/Protocols/Analytics#General_setup_of_enzyme_activity_measurements/ standard activity assay protocol] over night.]] | + | [[File:Bielefeld2012_new_TTHL_activity.jpg|500px|thumb|center|'''Figure 7''': Activity assay of each purified fraction of the new cultivation with TTHL. Samples were re-buffered into H<sub>2</sub>O and the protein amount in each fraction has been adjusted. The measurements were done using the [https://2012.igem.org/Team:Bielefeld-Germany/Protocols/Analytics#General_setup_of_enzyme_activity_measurements/ standard activity assay protocol] over night. The first number indicates the percentage of used elution buffer, whereas the second number stands for the fraction number of this elution.]] |
| + | ===Since Regionals: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL] activity depending on different ABTS concentrations=== | ||
| + | |||
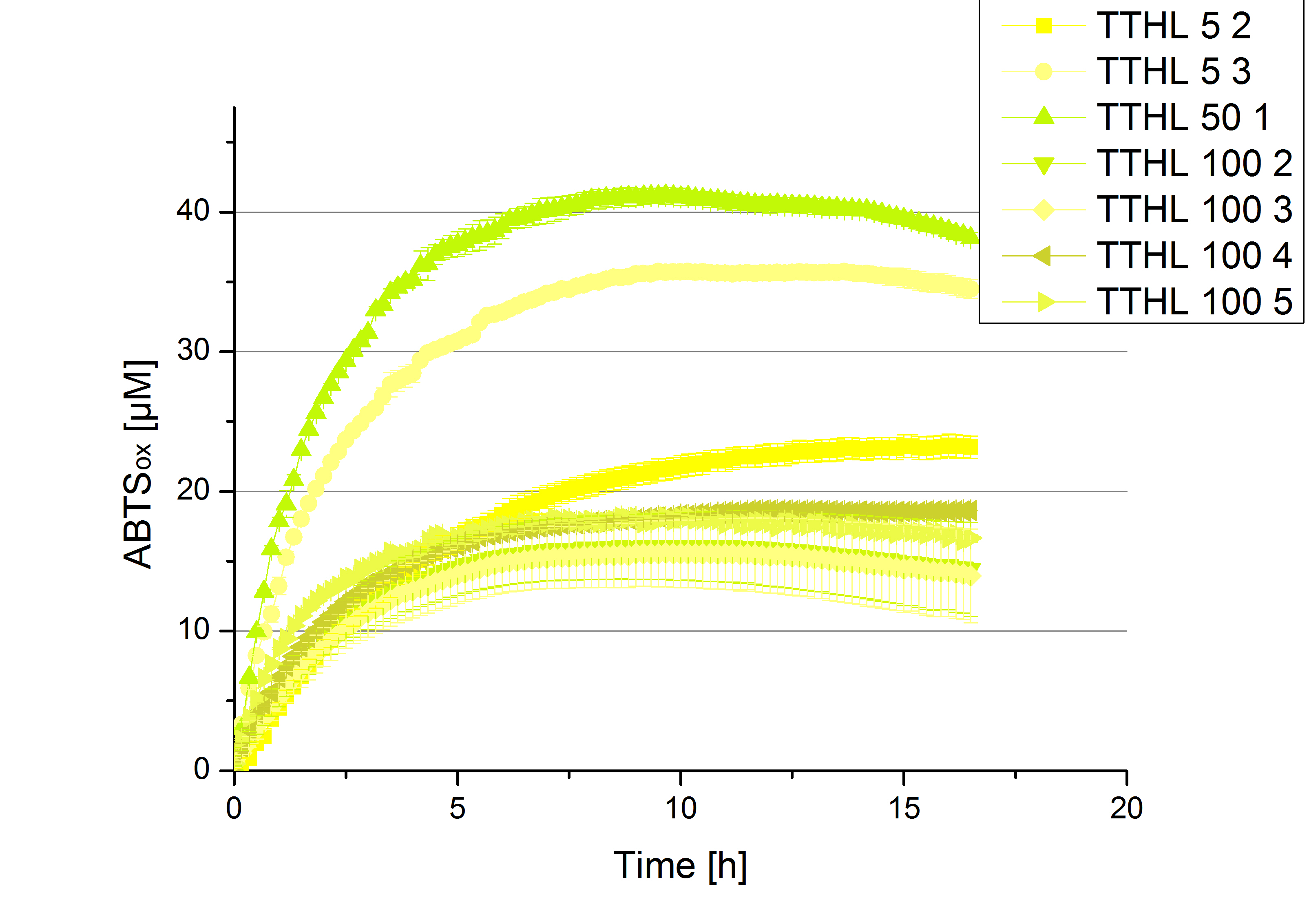
| + | In order to find the substrate saturation, laccase activity was measured with ABTS concentrations ranging from 0.1 mM to 8 mM. 616 ng TTHL laccase were used for measurements with ABTS concentrations of 0.1 mM to 5 mM, 308 ng TTHL laccase were used for measurements with ABTS concentrations of 5 mM to 8 mM. Measurements were done in Britton-Robinson buffer (pH 5) at 25 °C for 30 minutes taking the OD<sub>420</sub> every 5 minutes. Comparing the graphs in Figure 8 and Figure 9, the substrate saturation is not reached with 5 mM ABTS. An application of 8 mM shows less oxidized ABTS as measurements with 7 mM ABTS. Further experiments were done with 7 mM ABTS. | ||
| + | |||
| + | [[File:Bielefeld2012_TTHL_klein_ABTS.jpg|thumb|left|360px|'''Figure 8:''' Activity assay to determine the substrate saturation with ABTS as a substrate. Measurements were done with 616 ng TTHL laccase in Britton-Robinson buffer (pH 5) at 25 °C. ABTS concentrations ranged from 0.1 mM to 5 mM.]] | ||
| + | [[File:Bielefeld2012_TTHL_hoch.jpg|thumb|right|360px|'''Figure 9:''' Activity assay to determine the substrate saturation with ABTS as a substrate. Measurements were done with 308 ng TTHL laccase in Britton-Robinson buffer (pH 5) at 25 °C. ABTS concentrations ranged from 5 mM to 8 mM. An ABTS concentration of 7 mM was determined as substrate saturated.]] | ||
| + | |||
| + | |||
| + | <br style="clear: both" /> | ||
===Since Regionals: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL] pH optimum=== | ===Since Regionals: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL] pH optimum=== | ||
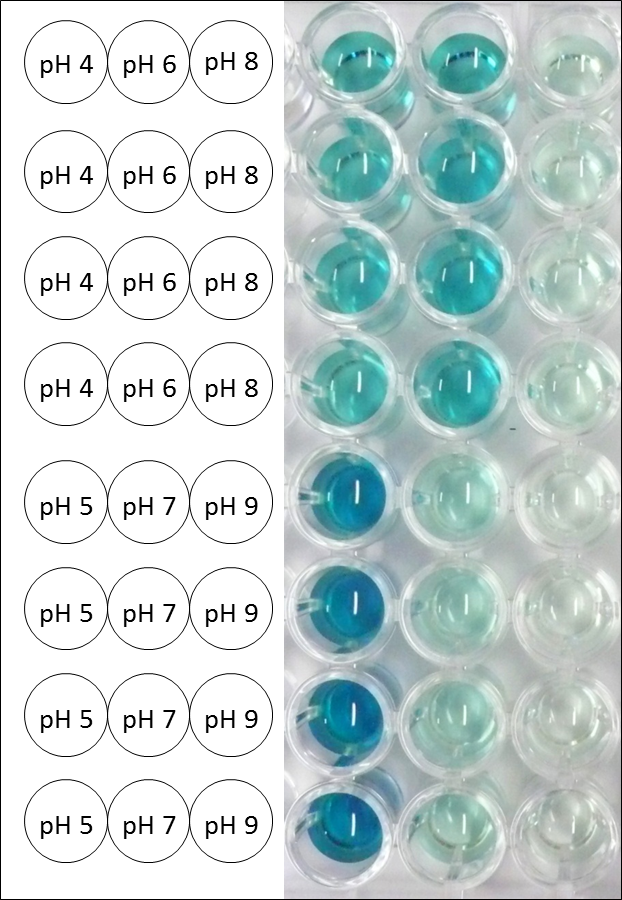
| - | [[File:Bielefeld2012_Thermo_pH_Foto.png|thumb|right|200px|Microtiter plate of the measurements for pH optimum determination. The more intensive the blue color the more ABTS got oxidized. At pH 5 the darkest | + | [[File:Bielefeld2012_Thermo_pH_Foto.png|thumb|right|200px|'''Figure 10:''' Microtiter plate of the measurements for pH optimum determination. The more intensive the blue color the more ABTS got oxidized. At pH 5 the darkest color has been reached.]] |
| + | |||
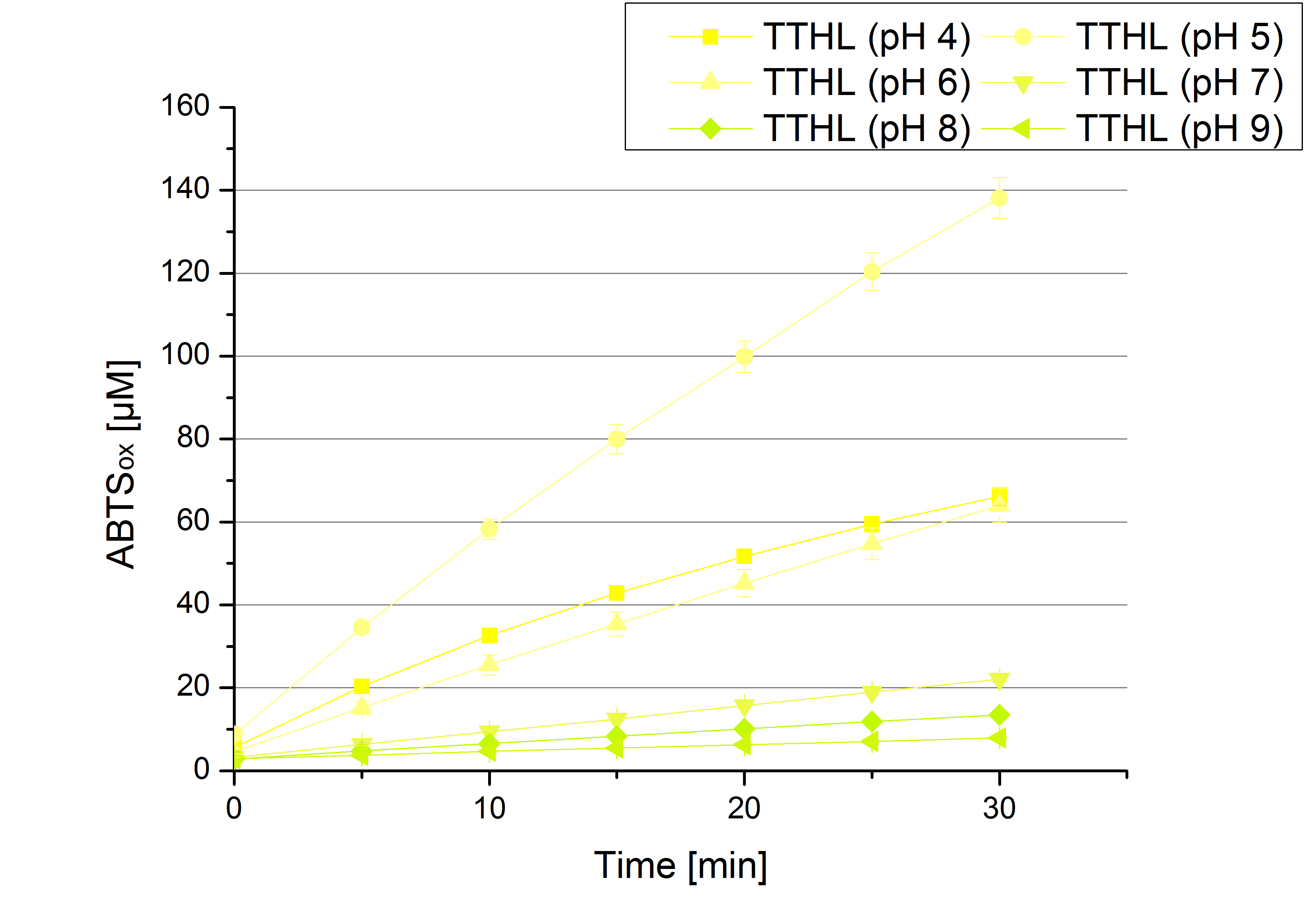
| + | The pH of the medium containing the enzyme is very important for its activity. The pH optimum of TTHL is at pH 5. This is the result of activity measurements using Britton-Robinson buffer with differently adjusted pHs. TTHL laccase was re-buffered into H<sub>2</sub>O and incubated with 0.4 mM CuCl<sub>2</sub>. The range from pH 4 to pH 9 was tested under substrate saturation at 25 °C for 30 minutes. At pH 5 ABTS gets oxidized the fastest (see Fig. 10 and 11). At higher and lower pHs than pH 5, the activity of TTHL is decreased considerably. The resulting Units mg<sup>-1</sup> support the observed data (see Fig. 12). At pH 5 TTHL shows a specific enzyme activity of ~15 U mg<sup>-1</sup>. The higher the pH, the less U mg-1 can be calculated for TTHL. At pH 4 and 6 the activity is decreased to 42 % and at pH 7 even to 14 % in comparison to pH 5. But still TTHL is active at this pH allowing an application of this laccase in a waste water treatment plant where the average pH is a pH of 6.9. However, a combination with a more effective enzyme should be considered. | ||
| + | <br style="clear: both" /> | ||
| + | [[File:Bielefeld2012_TTHL_pH_new.jpg|thumb|left|360px|'''Figure 11:''' Oxidized ABTS by TTHL laccases at different pH adjustments. The experimental setup included CuCl<sub>2</sub> incubated TTHL laccase (308 ng), Britton Robinson buffer adjusted to the tested pHs and 5 mM ABTS. Measurements were done at 25 °C for 30 minutes. The most amount of oxidized ABTS can be detected at pH 5.]] | ||
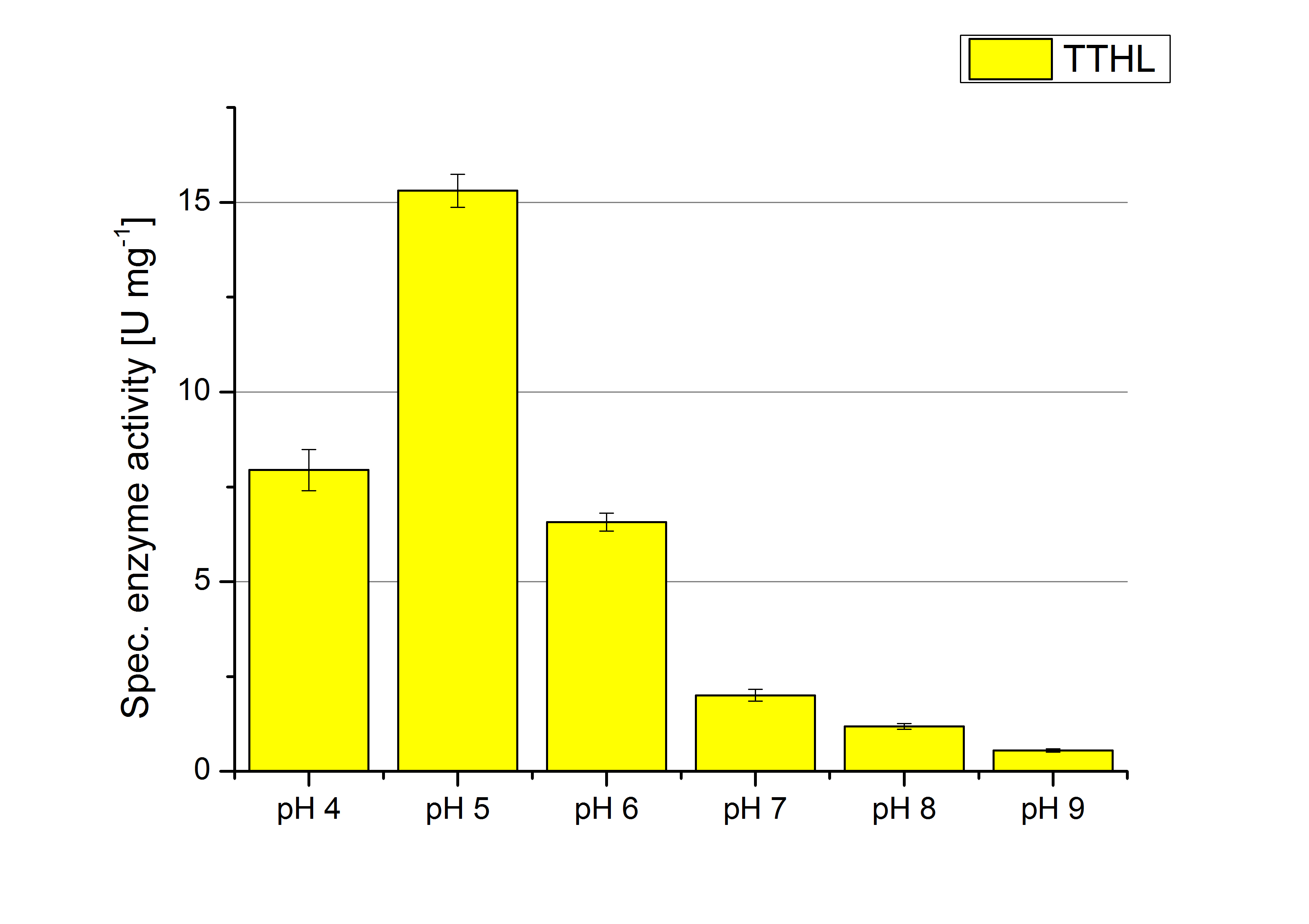
| + | [[File:Bielefeld2012_TTHL_pH_Units.jpg|thumb|right|360px|'''Figure 12:''' Calculated specific enzyme activity of TTHL at different pH conditions. The highest specific enzyme activity for ABTS is under pH 5 conditions. The higher the pH, the less ABTS gets oxidized. One unit is defined as the amount of laccase that oxidizes 1 μmol of ABTS substrate per minute.]] | ||
<br style="clear: both" /> | <br style="clear: both" /> | ||
===Since Regionals: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL] activity at different temperatures=== | ===Since Regionals: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL] activity at different temperatures=== | ||
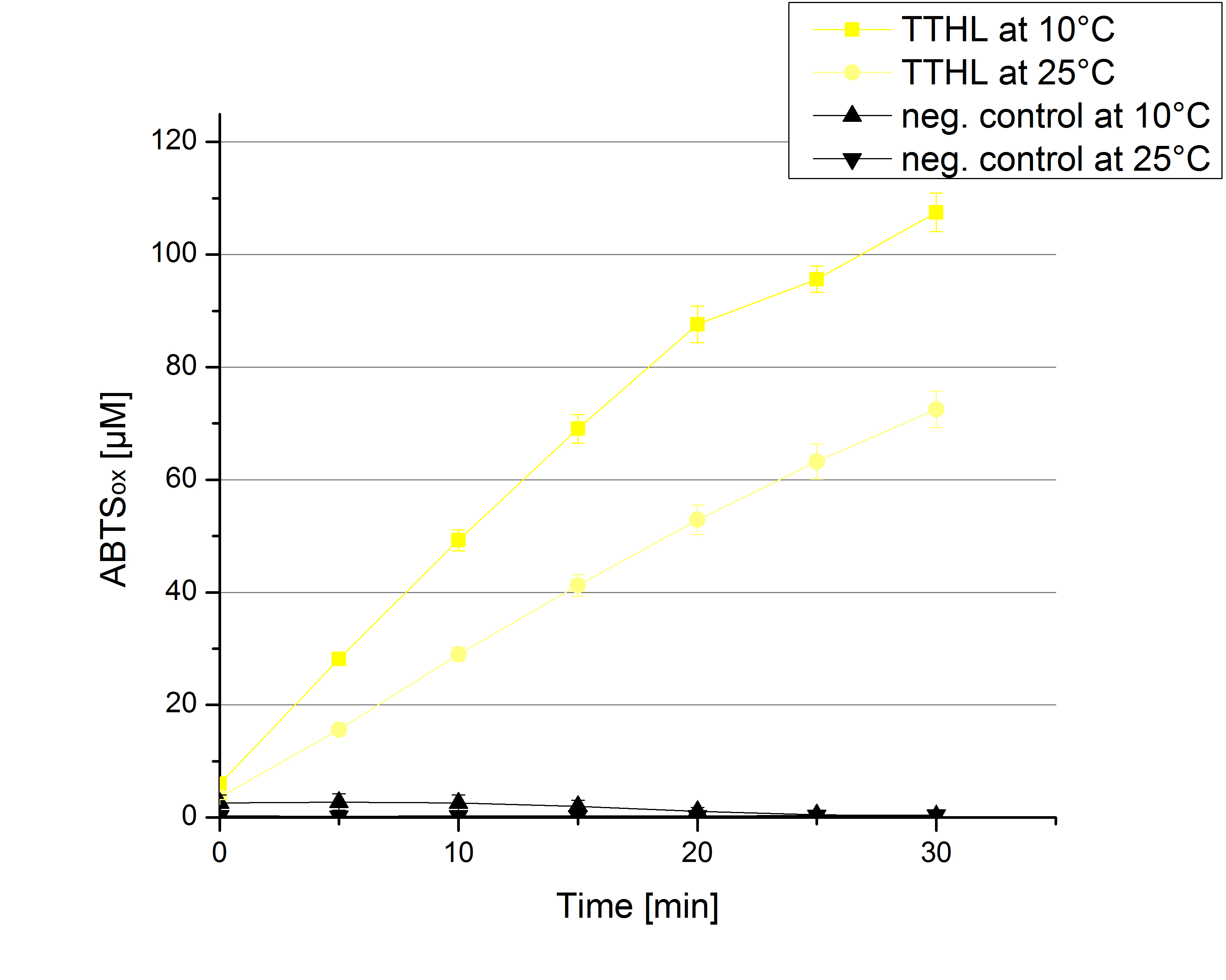
| + | [[File:Bielefeld2012 TTHL Temp ABTSox.jpg|left|200px|thumb|'''Figure 13''': Standard activity test for TTHL measured at 10 °C and 25 °C resulting in a decreased activity at 10 °C. As a negative control the impact of 0.4 mM CuCl<sub>2</sub> in oxidizing ABTS at 10 °C and 25 °C were analyzed.]] | ||
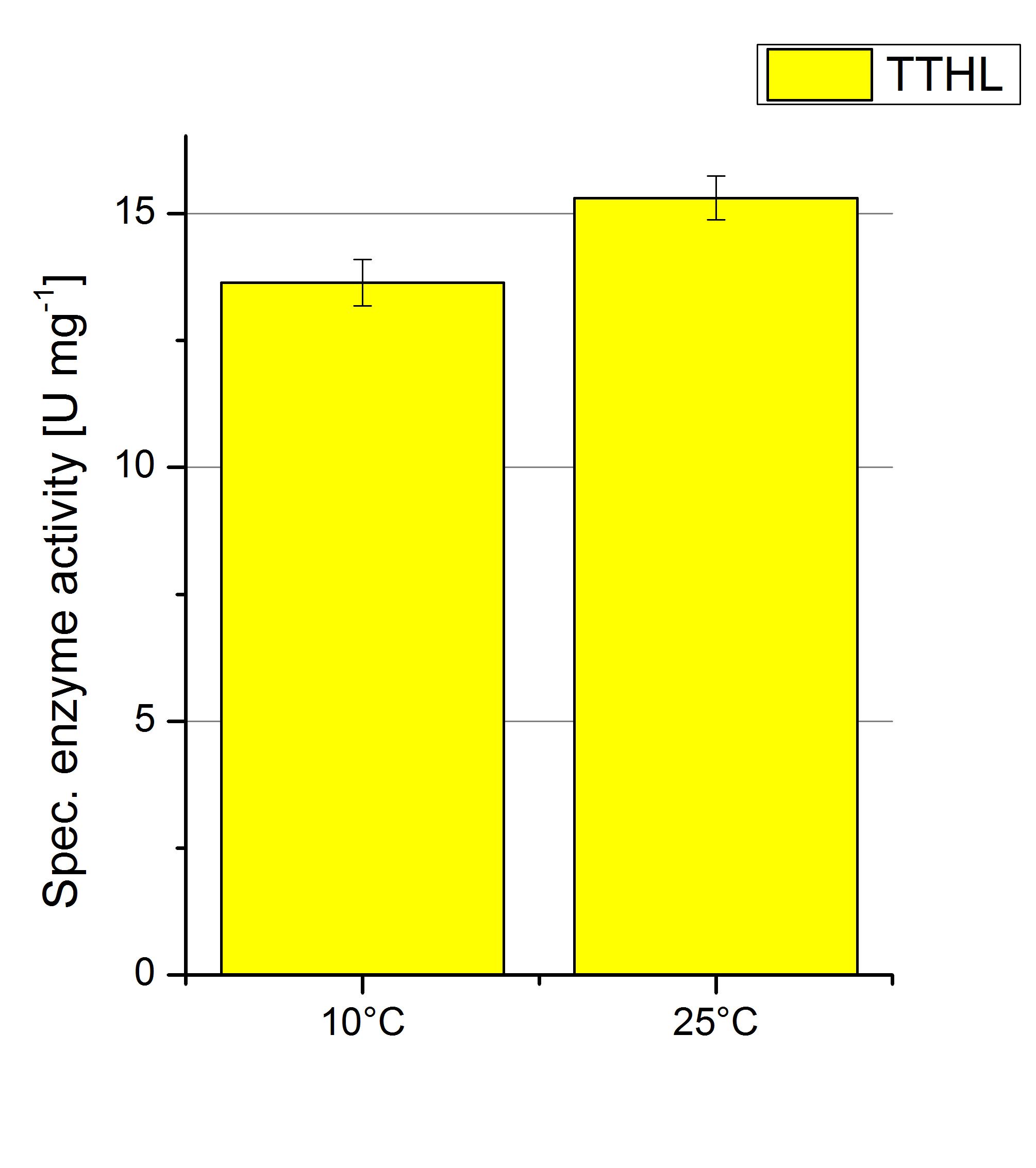
| + | [[File:Bielefeld2012 TTHL Temp Units.jpg|right|200px|thumb|'''Figure 14''': Deriving from the obtained values of oxidized ABTS in time at 10 °C and 25 °C the specific enzyme activity was calculated. For the temperatures a difference of 2 U mg<sup>-1</sup> could be detected. One unit is defined as the amount of laccase that oxidizes 1 μmol of ABTS substrate per minute.]] | ||
| - | + | To investigate the activity of TTHL at temperatures that will apply at a waste water treatment plant throughout the year, activity tests as described above were performed at 10 °C and 25 °C. The measurements were conducted for 30 minutes. The obtained results reveal an activity decrease of about 35 % of TTHL at 10 °C in comparison to 25 °C (see Fig. 13). The obtained results were used to calculate the specific enzyme activity which was at 13 and 15 U mg<sup>-1</sup>, respectively (see Fig. 14). The negative control without TTHL laccase but 0.4 mM CuCl<sub>2</sub> at 10 °C and 25 °C show a negligible oxidation of ABTS. The low difference observed between the two samples is great news for the possible application in waste water treatment plants. | |
| + | <br><br><br><br><br><br><br><br><br> | ||
| + | == Immobilization == | ||
| + | |||
| + | |||
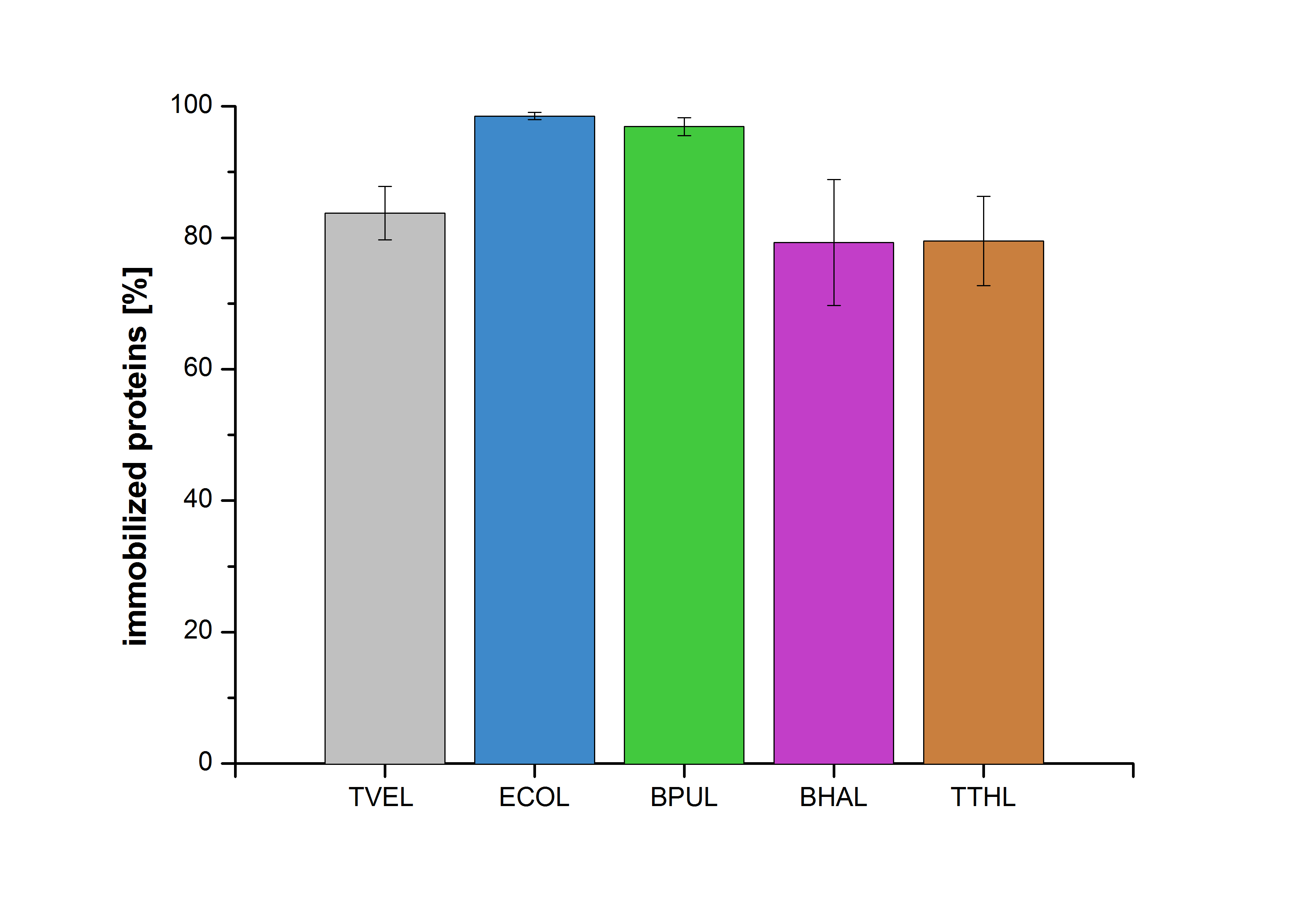
| + | [[File:Bielefeld2012-Immobilized_proteins.jpg|500px|left|thumb|'''Figure 15''': The percentage of laccases immobilized to CPC-Beads. 99 % of ECOL, 97 % of BPUL and 79 % of BHAL and TTHL laccases were bound to the beads.]] | ||
| + | |||
| + | <div style="text-align:justify;"> | ||
| + | Figure 15 shows the percentage of laccases bound after incubation with CPC-beads, relative to the original concentration. The concentration of laccases in the supernatant after incubation was measured using Roti®-Nanoquant. The results showed that only 21% of TTHL laccases was still present in the supernatant. This illustrates that TTHL was successfully immobilized on the CPC-beads. | ||
| + | </div> | ||
| + | <br style="clear: both" /> | ||
| + | |||
| + | |||
| + | |||
| + | [[File:Bielefeld2012-Graphen_Bead_Thermo.jpg|500px|left|thumb|'''Figure 16''': Illustration of ABTS oxidation by TTHL with time compared to the negative control. The increase in ABTS oxidized proves laccase activity.]] | ||
| + | <div style="text-align:justify;"> | ||
| + | Figure 16 shows the illustration of ABTS oxidation by TTHL with time compared to the negative control. The increase in ABTS oxidized proves laccase activity even if a direct comparison with the original and not immobilized laccase solution was not possible due to the very low concentration of purified TTHL. | ||
| + | <br style="clear: both" /> | ||
| + | </div> | ||
| + | ==Substrate Analysis== | ||
| + | |||
| + | [[File:Bielefeld2012_Ohne_ABTS.png|400px|thumb|right|'''Figure 17:''' Degradation of estradiol (light green) and ethinyl estradiol (dark green) with the different laccases after 5 hours without ABTS. In the graph it is shown that the bought laccase TVEL0 which was used as positive control is able to degrade more than 90 percent of the used substrates. None of the bacterial laccases are able to degrade ethinyl estradiol without ABTS but estradiol is degraded in a range from 16 %(ECOL) to 55 % (TTHL). The original concentrations of substrates were 2 µg per approach. (n = 4)]] | ||
| + | |||
| + | |||
| + | The measurements were made to test if the produced laccases were able to degrade different hormones. Therefore the produced laccases were inserted in the same concentrations (3 µg mL<sup>-1</sup>) to the different measurement approaches. To work with the correct pH value (which were measured by the Team Activity Test) Britton Robinson buffer at pH 5 was used for all measurements. The initial substrate concentration was 5 µg mL<sup>-1</sup>. The results of the reactions without ABTS are shown in Figure 17. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are indicated. The X-axis displays the different tested laccases. The degradation was measured at t<sub>0</sub> and after five hours of incubation at 30 °C. The negative control was the substrate in Britton Robinson buffer and showed no degradation of the substrates. The bought laccase TVEL0 which is used as positive control is able to degrade 94.7 % estradiol and 92.7 % ethinyl estradiol. The laccase BPUL (from ''Bacillus pumilus'') degraded 35.9 % of used estradiol after five hours. ECOL was able to degrade 16.8 % estradiol. BHAL degraded 30.2 % estradiol. The best results were determined with TTHL (laccase from ''Thermus thermophilus''). Here the percentage of degradation amounted 55.4 %. | ||
| + | |||
| + | [[File:Bielefeld2012_Mit_ABTS.png|400px|thumb|left|'''Figure 18:''' Degradation of estradiol (light green) and ethinyl estradiol (dark green) with the different laccases after 10 minutes hours with ABTS added. The commercial laccase TVEL0 which was used as positive control is able to degrade all of the used substrates. The bacterial laccase BPUL degraded 100 % of ethinyl estradiol and estradiol. ECOL the laccase from ''E. coli'' degraded 6.7 % estradiol and none of the used ethinyl estradiol. BHAL degraded 46.9 % of estradiol but no ethinyl estradiol. The laccase TTHL from ''Thermus thermophilus'' degraded 29.5 % of estradiol and 9.8 % ethinyl estradiol. The original concentrations of substrates were 2 µg per approach. (n = 4)]] | ||
| + | |||
| + | The results of the reactions of the laccases with addition of ABTS are shown in Figure 18. The experimental set ups were the same as the reaction approach without ABTS described above. The X-axis displays the different tested laccases. On the Y-axis the percentages of degraded estradiol (light green) and ethinyl estradiol (dark green) are shown. The degradation was measured at t<sub>0</sub> and after five hours of incubation at 20 °C. The negative control showed no degradation of estradiol. 6.8 % of ethinyl estradiol was decayed. The positive control TVEL0 is able to degrade 100 % estradiol and ethinyl estradiol. The laccase BPUL (from ''Bacillus pumilus'') degraded 46.9 % of used estradiol after ten minutes incubation. ECOL was able to degrade 6.7 % estradiol. BHAL degraded 46.9 % estradiol. With TTHL (laccase from ''Thermus thermophilus'') a degradation 29.5 % were determined. | ||
| + | <br style="clear: both" /> | ||
| + | |||
| + | <html> | ||
| Line 125: | Line 179: | ||
{{Team:Bielefeld/Sponsoren}} | {{Team:Bielefeld/Sponsoren}} | ||
/partinfo>. The maximal OD | /partinfo>. The maximal OD | ||
| + | /div> | ||
| + | |||
| + | ===Since Regionals: Purification of TTHL since Regionals=== | ||
Latest revision as of 13:46, 6 March 2013

Summary
Initially some trials of shaking flask cultivations were made with different parameters to identify the best conditions for the production of the His-tagged laccase LttH from [http://www.dsmz.de/catalogues/details/culture/DSM-7039.html?tx_dsmzreso Thermus thermophilus HB27] named TTHL. Due to the absence of enzyme activity of the enzyme in the cell lysate a purification method was established (using Ni-NTA-His tag resin). Using E. coli KRX containing BioBrick <partinfo>BBa_K863010</partinfo>, TTHL could not be detected by SDS-PAGE (molecular weight of 53 kDa) or by activity test. Therefore a new BioBrick <partinfo>BBa_K863012</partinfo> was constructed and expressed in E. coli Rosetta-Gami 2. With this expression system the TTHL could be detected by SDS-PAGE and purified by using a small scale Ni-NTA column. The fractionated samples were tested regarding their activity. TTHL was shown to oxidize ABTS. After measuring activity of TTHL a scale up of the fermentation was successfully implemented up to 6 L. A further scale up to 12 L with a optimized medium (HSG) and a labscale Ni-NTA-Purification were implemented to enable additional experiments to characterize TTHL. A total specific enzyme activity of 15 U mg-1 was determined for TTHL at pH 4 at 25°C with ABTS as a substrate.
Cultivation, Purification and SDS-PAGE
Shaking Flask Cultivation
The first trials to produce the LttH-laccase from [http://www.dsmz.de/catalogues/details/culture/DSM-7039.html?tx_dsmzresources_pi5 Thermo thermophilus HB27] (named TTHL) were performed in shaking flasks with various volumes (from 100 mL up to 1 L flasks, with and without baffles) and under different cultivation conditions. The best cultivation condition for <partinfo>BBa_K863010</partinfo> expressed in E. coli was screened by varying the temperature, the chloramphenicol concentration,induction strategy and cultivation time. Furthermore, E. coli was cultivated with and without 0.25 mM CuCl2 in the medium to provide a sufficient amount of copper, which is needed for bilding the active center. Under the screened conditions no biological active TTHL could be produced. Therefore another BioBrick was constructed and another chassi was chosen. To improve the expression another BioBrick <partinfo>BBa_K863012</partinfo> was used, which has a constitutive promoter instead of the T7 promoter system. Additionally, the strain E. coli Rosetta-Gami 2 was chosen, because of its ability to translate rare codons. TTHL was then produced under the following conditions:
- flask design: shaking flask without baffles
- medium: LB-Medium
- antibiotics: 60 µg mL-1 chloramphenicol and 300 µg mL-1 ampicillin
- temperature: 37 °C
- cultivation time: 24 h
The reproducibility of the measured data and results were investigated for the shaking flask cultivation, but not yet for the bioreactor cultivation.
Fermentation of E. coli KRX with <partinfo>BBa_K863012</partinfo>
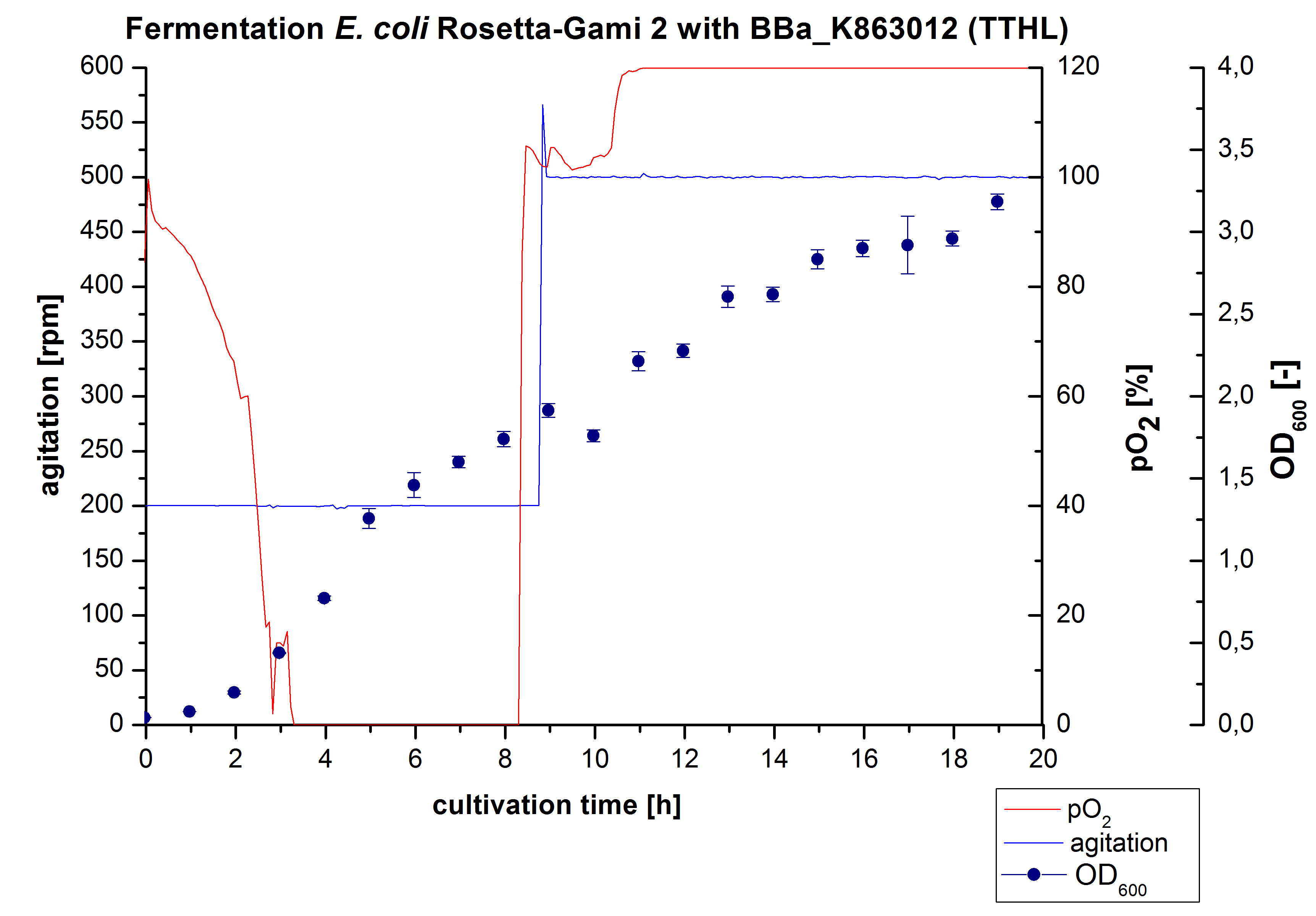
After measuring activity of TTHL we made a scale-up and cultivated E. coli Rosetta-Gami 2 expressing <partinfo>BBa_K863000</partinfo> in a Bioengineering NFL22 fermenter with a total volume of 6 L. Agitation speed, pO2 and OD600 were online monitored and are illustrated in Figure 1. No initial lag phase was noticeable. Due to the cell growth the pO2 decreased,breakdown of the control unit resulted in a drop to 0%. After a cultivation time of 9 hours the agitation speed was therefore increased manually up to 500 rpm, which resulted in a higher pO2 value of more than 100 % for the rest of the cultivation. During the whole process the OD600 increased slower compared to the fermentation of E. coli KRX expressing <partinfo>BBa_K863000</partinfo> or <partinfo>BBa_K863005</partinfo>. The maximal OD600 was reached after 19 hours cultivation time at which point the cells were harvested.
Purification of TTHL
The cells were harvested by centrifugation and resuspended in Ni-NTA-equilibrationbuffer, mechanically disrupted by high pressure homogenization and centrifuged. After preparing the cell paste the TTHL could not be purified with the 15 mL column, due to a not available column. For this reason a small scale purification (6 mL) of the supernatant of the homogenisation was made with a 1 mL Ni-NTA-column.
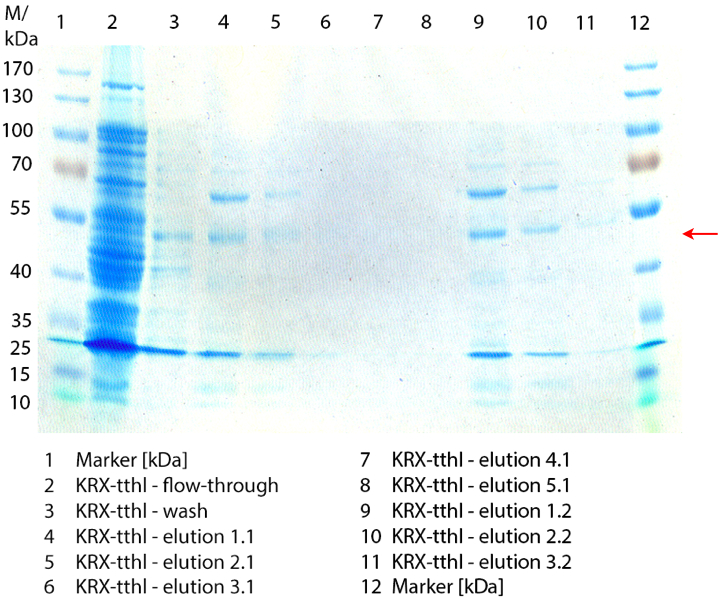
SDS-PAGE of purified TTHL
Figure 2 shows the SDS-PAGE of the purified E. coli Rosetta-Gami 2 lysates fermented in 6 L Bioengineering NFL22 fermenter. Additionally the flow-through, wash and all elution fractions (1 to 5) are shown. TTHL has a molecular weight of 53 kDa and the corresponding band is marked with a red arrow. The TTHL band can be found in fractions 1 to 3, but not in the other two elution fractions. Furthermore there are some other non-specific bands, which could not be identified. To improve the purification an 15 mL column was implemented.
Since Regionals: 12 L Fermentation of E. coli Rosetta Gami 2 with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 BBa_K863012]
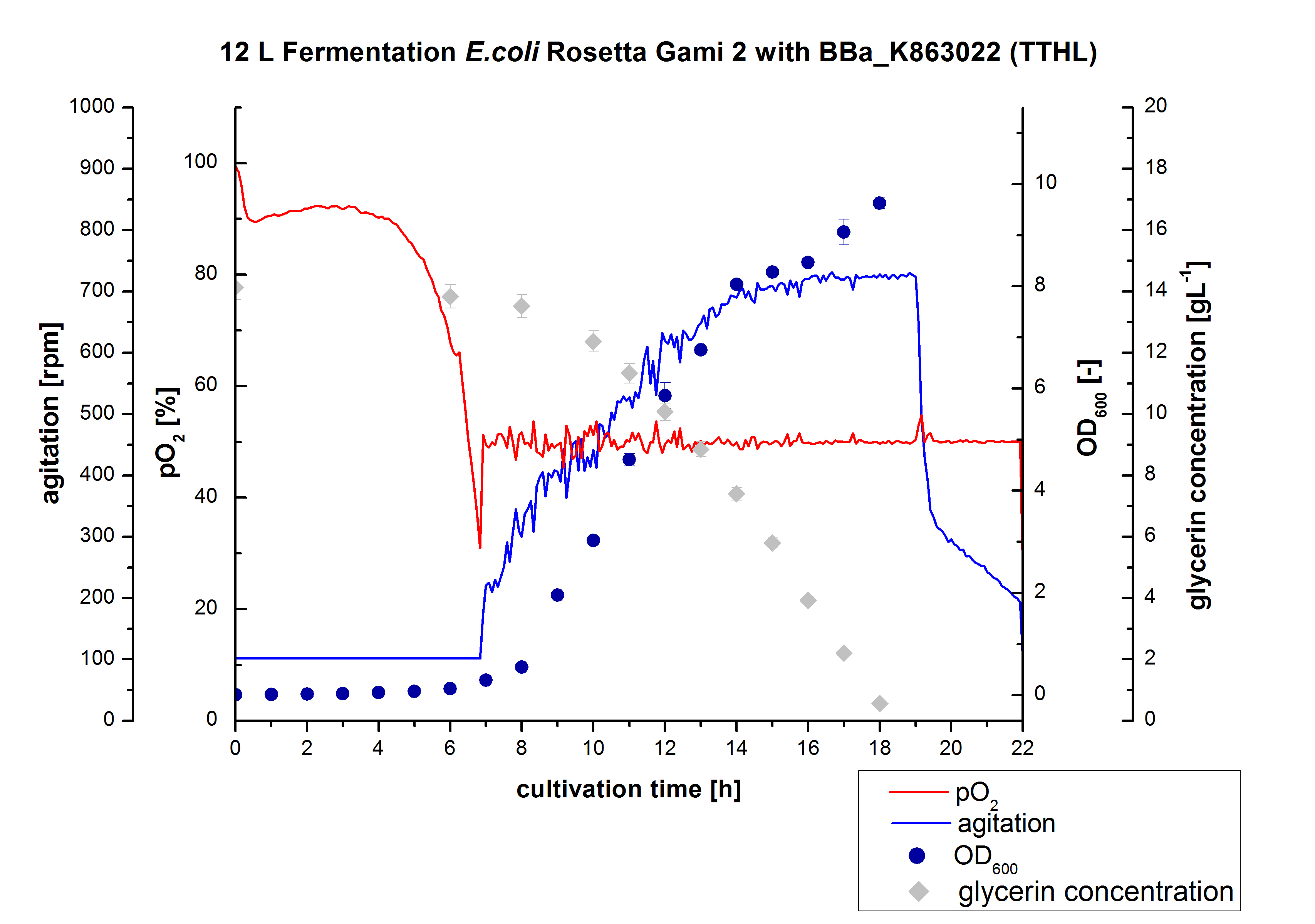
Another scale-up of the fermentation of E. coli Rosetta-Gami 2 with <partinfo>BBa_K863012</partinfo> was made up to a final working volume of 12 L in a Bioengineering NFL 22 fermenter. Agitation speed, pO2 and OD600 were determined as well as the glycerin concentration. The data are illustrated in Figure 3. This time HSG autodinduction medium was used to produce more biomass. Due to the change of media and to a low amount of cells for inocculation, there was a long lag phase of about 6 hours. During this phase the glycerin concentration is nearly constant. The cells were in an exponential phase between 8 and 18 hours of cultivation, which results in a decrease of gylcerin, of pO2 value and therefore in an increase of agitation speed. After 18 hours of cultivation the maximal OD600 of 9.63 was reached and the glycerin was completely consumed. At that time the cells were just entering the stationary phase. No further data for OD600 were measured. The cells have been harvested after 22 hours of cultivation. In the review, to leave the cells longer in the stationary phase could have been a better procedure concerning the yield.
Since Regionals: Purification of TTHL since Regionals
The harvested cells were resuspended in Ni-NTA- equilibration buffer and mechanically disrupted by homogenization. The cell debris were removed by centrifugation and microfiltration via [http://www.millipore.com/catalogue/module/C7493 Millipore Pellicon XL 50]. The supernatant of the cell lysate was concentrated with [http://www.millipore.com/catalogue/module/C7493 Millipore Pellicon XL 50] with 10 kDa and loaded on the Ni-NTA column (15 mL Ni-NTA resin) with a flow rate of 1 mL min-1 cm-2. Then the column was washed with 10 column volumes (CV) Ni-NTA equilibration buffer. The bound proteins were eluted by an increasing Ni-NTA elution buffer step elution from 5 % (equates to 25 mM imidazole) with a length of 50 mL, to 50 % (equates to 250 mM imidazole) with a length of 80 mL and finally to 100 % (equates to 500 mM imidazole) with a length of 80 mL. This strategy was chosen to improve the purification caused by a step by step increasing Ni-NTA-elution buffer concentration. The elution was collected in 10 mL fractions. In Figure 3 only the UV-detection signal of the wash step and the elution are shown, this is because of the high UV-detection signal of the loaded samples and to simplify the illustration of the detected product peak. A typical chromatogram of purified laccases is illustrated here. The chromatogram of the TTHL elution is shown in Figure 4:
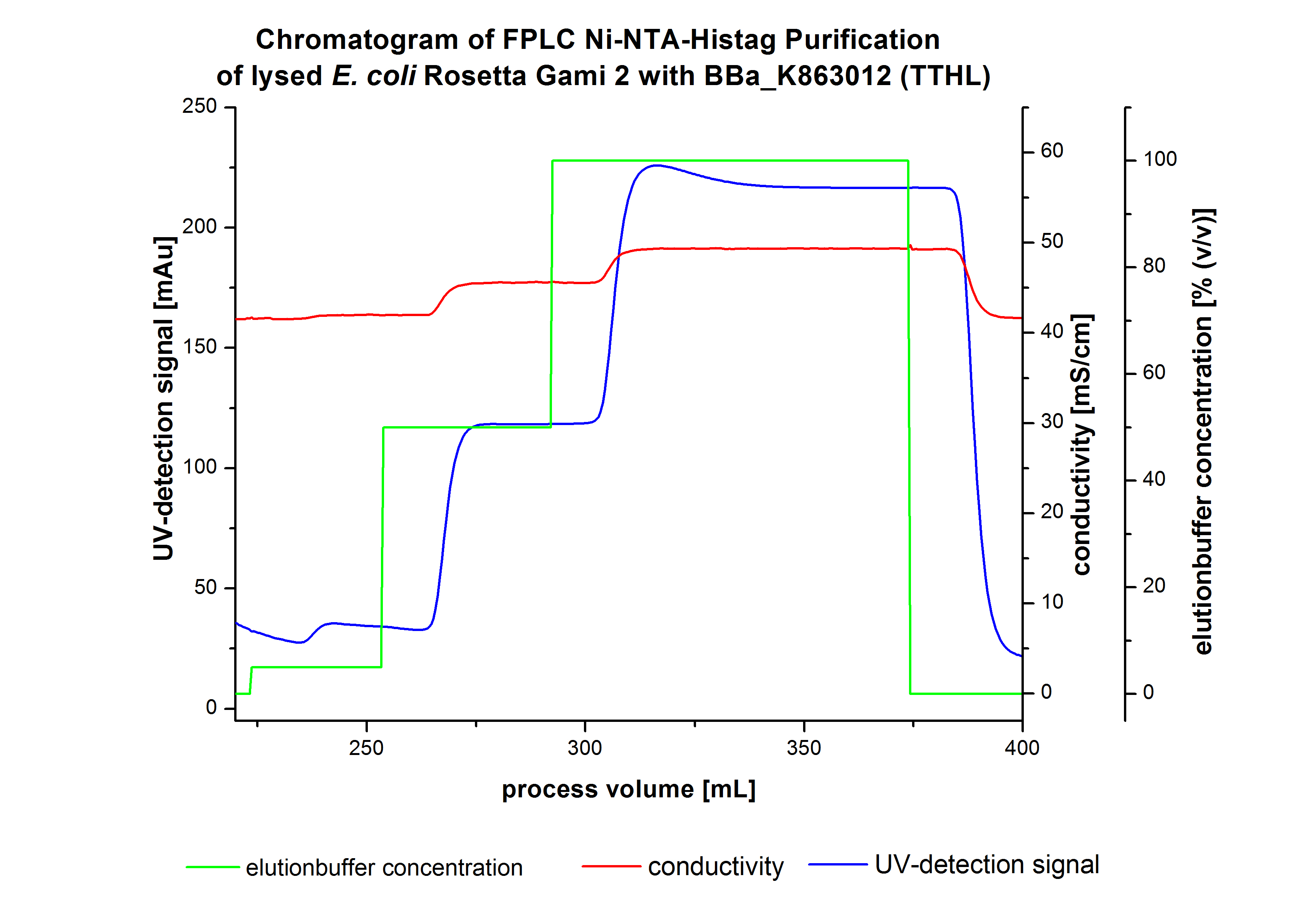
Contrary to our expectations, the chromatogram shows one distinguished peak. This peak was detected at a Ni-NTA-equilibration buffer concentration of 100 % (equates to 500 mM imidazole) and resulted from the elution of bound protein. Earlier measurements of other bacterial laccases showed that the elution of these laccases begins with a elution buffer concentration of 50 % equates to 250 mM imidazole. One explanation of this result could be a low concentration of the produced TTHL. Consequently all elution fractions were analyzed by SDS-PAGE to detect TTHL. In the chromatogram no further peaks were detected. The following increasing UV detection signal by increasing concentration of the elution buffer results from the rising imidazole concentration of the Ni-NTA elution buffer. The corresponding SDS-PAGES are shown in Figure 5.
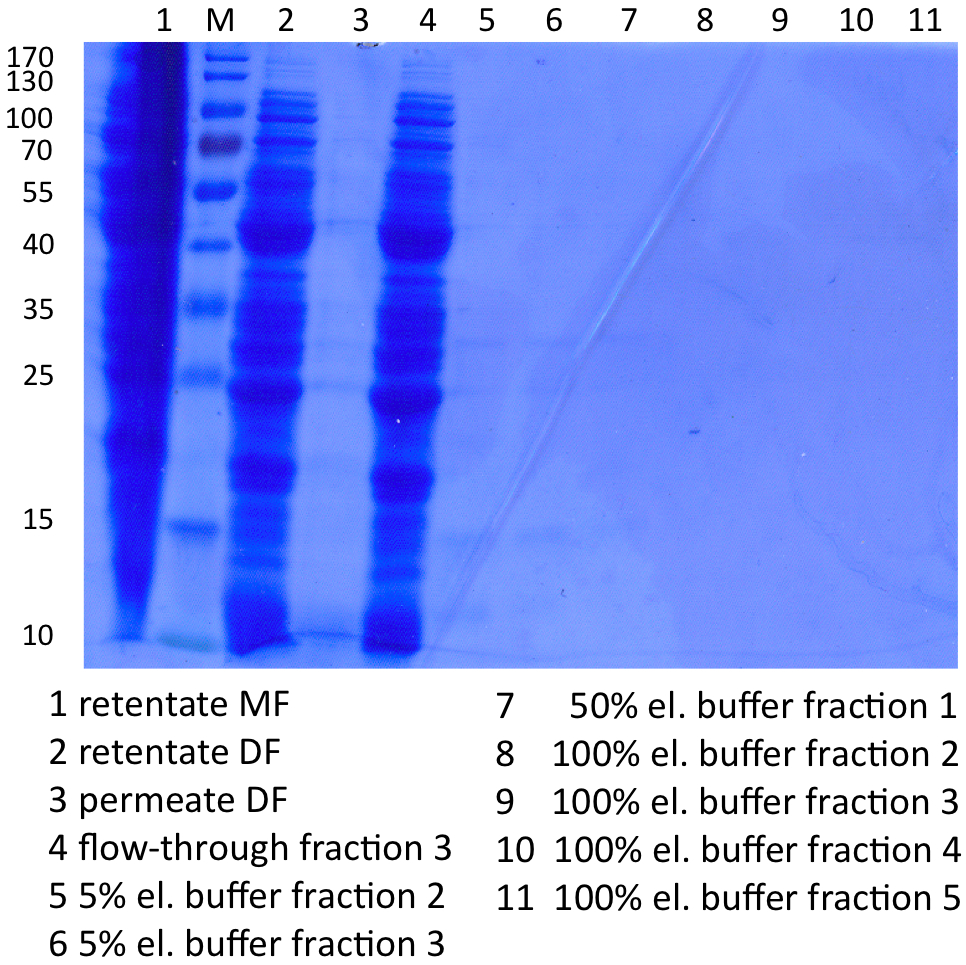
Since Regionals: SDS-PAGE of protein purification
In Figure 5 the SDS-PAGE of the Ni-NTA purification of the lysed E.coli Rosetta-Gami 2 culture containing [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 BBa_K863012] is illustrated. It shows the permeate and retentate of microfiltration and diafiltration respectively, several fractions of flow-through, wash and the elutions with different buffer concentrations respectively. The selected samples were taken where peaks were seen in the chromatogram. The His-tagged TTHL has a molecular weight of 53 kDa. Apparently the concentration of TTHL is too low to see a band.
Activity analysis of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL]
Initial activity tests of purified fractions
There was no activity measurable after cultivation and purification of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863022 BBa_K863022] under the control of a T7 promoter. Activity tests of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL] under a constitutive promoter did reveal TTHL laccases capable of oxidizing ABTS. Fractions 1 to 5 of the purification above were rebuffered with deionized H2O and incubated with 0.4 mM CuCl2 for 2 hours. Activity measurements were performed using 140 µL sample, 0.1 mM ABTS and 100 mM sodium acetate buffer (pH 5) to a final volume of 200 µL. The change in optical density at 420 nm was detected, reporting the oxidization of ABTS through laccases. Fractions 1 to 5 show activity (Figure 6). Fraction 2 seems to contain most of TTHL showing the highest activity compared to the other fractions: 40 % of the used ABTS has been oxidized after 2 hours. Based on these results protein concentrations have to be determined and the activity of the TTHL laccase can be characterized in further experiments including pH optimum and activity in regard of temperature shifts.
Since Regionals: Initial activity tests of purified fractions
The purificated fractions of the cultivation after the Regional Jamborees in Amsterdam were tested concerning their protein concentration. After re-buffering the protein concentration was determined again and all fraction were incubated with 0.4 mM CuCl2. For the initial activity test the protein amount was adjusted for comparison. The fractions were measured in Britton-Robinson buffer at pH 5 with 0.1 mM ABTS. Fraction 50 % 1 showed the highest activity (Fig. 7). Regarding the protein amount of this fraction and the statement, that 90 % of this are TTHL laccase, fraction 50 % 1 contains 4,03 µg mL-1. To ensure enough protein for further experiments, the second best fraction, which is fraction 5 % 3 was added to fraction 50 % 1. The first number indicates the percentage of used elution buffer, whereas the second number stands for the fraction number of this elution. In total, both fraction contain 4,4 µg mL-1.
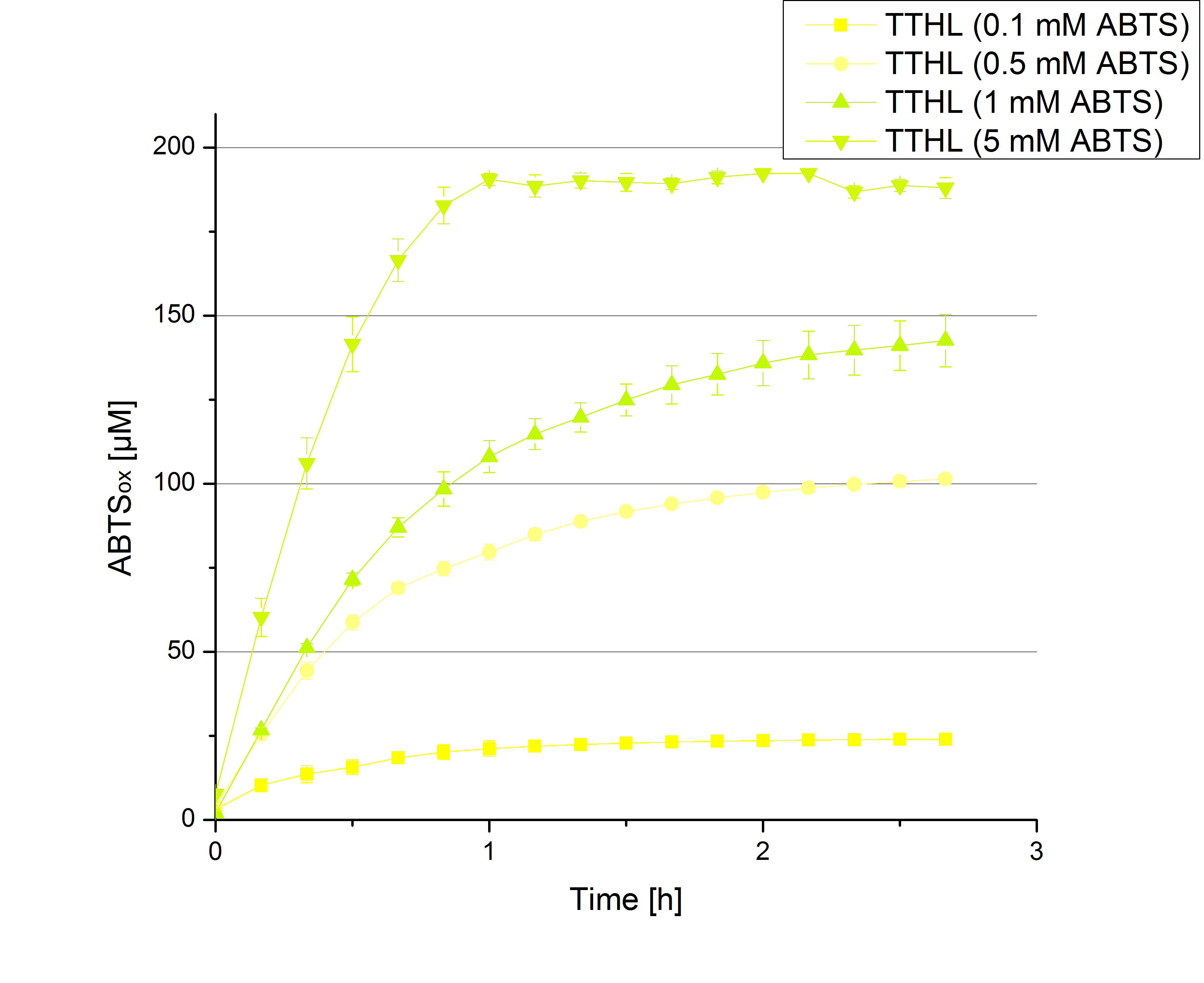
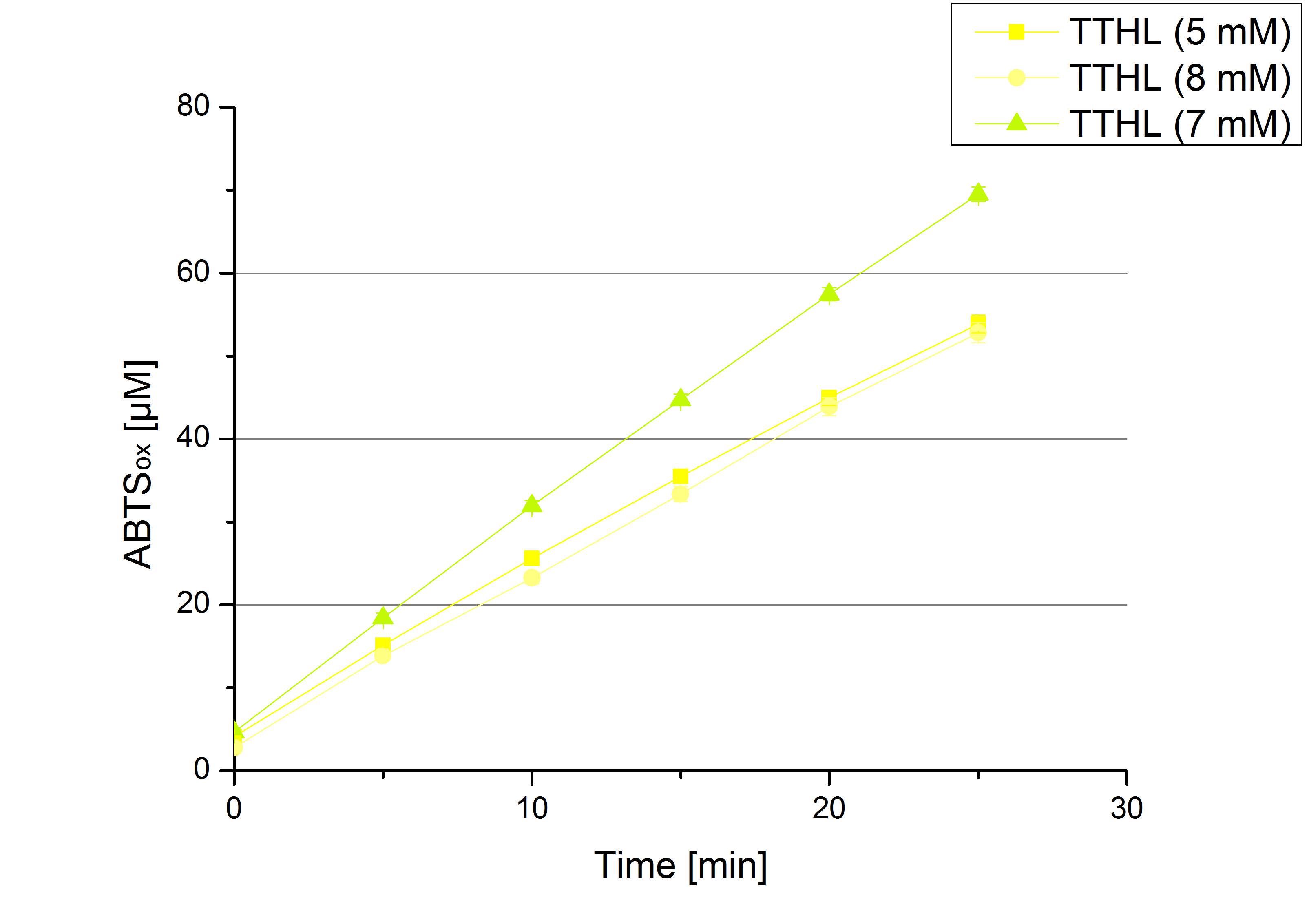
Since Regionals: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL] activity depending on different ABTS concentrations
In order to find the substrate saturation, laccase activity was measured with ABTS concentrations ranging from 0.1 mM to 8 mM. 616 ng TTHL laccase were used for measurements with ABTS concentrations of 0.1 mM to 5 mM, 308 ng TTHL laccase were used for measurements with ABTS concentrations of 5 mM to 8 mM. Measurements were done in Britton-Robinson buffer (pH 5) at 25 °C for 30 minutes taking the OD420 every 5 minutes. Comparing the graphs in Figure 8 and Figure 9, the substrate saturation is not reached with 5 mM ABTS. An application of 8 mM shows less oxidized ABTS as measurements with 7 mM ABTS. Further experiments were done with 7 mM ABTS.
Since Regionals: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL] pH optimum
The pH of the medium containing the enzyme is very important for its activity. The pH optimum of TTHL is at pH 5. This is the result of activity measurements using Britton-Robinson buffer with differently adjusted pHs. TTHL laccase was re-buffered into H2O and incubated with 0.4 mM CuCl2. The range from pH 4 to pH 9 was tested under substrate saturation at 25 °C for 30 minutes. At pH 5 ABTS gets oxidized the fastest (see Fig. 10 and 11). At higher and lower pHs than pH 5, the activity of TTHL is decreased considerably. The resulting Units mg-1 support the observed data (see Fig. 12). At pH 5 TTHL shows a specific enzyme activity of ~15 U mg-1. The higher the pH, the less U mg-1 can be calculated for TTHL. At pH 4 and 6 the activity is decreased to 42 % and at pH 7 even to 14 % in comparison to pH 5. But still TTHL is active at this pH allowing an application of this laccase in a waste water treatment plant where the average pH is a pH of 6.9. However, a combination with a more effective enzyme should be considered.
Since Regionals: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 TTHL] activity at different temperatures
To investigate the activity of TTHL at temperatures that will apply at a waste water treatment plant throughout the year, activity tests as described above were performed at 10 °C and 25 °C. The measurements were conducted for 30 minutes. The obtained results reveal an activity decrease of about 35 % of TTHL at 10 °C in comparison to 25 °C (see Fig. 13). The obtained results were used to calculate the specific enzyme activity which was at 13 and 15 U mg-1, respectively (see Fig. 14). The negative control without TTHL laccase but 0.4 mM CuCl2 at 10 °C and 25 °C show a negligible oxidation of ABTS. The low difference observed between the two samples is great news for the possible application in waste water treatment plants.
Immobilization
Figure 15 shows the percentage of laccases bound after incubation with CPC-beads, relative to the original concentration. The concentration of laccases in the supernatant after incubation was measured using Roti®-Nanoquant. The results showed that only 21% of TTHL laccases was still present in the supernatant. This illustrates that TTHL was successfully immobilized on the CPC-beads.
Figure 16 shows the illustration of ABTS oxidation by TTHL with time compared to the negative control. The increase in ABTS oxidized proves laccase activity even if a direct comparison with the original and not immobilized laccase solution was not possible due to the very low concentration of purified TTHL.
Substrate Analysis
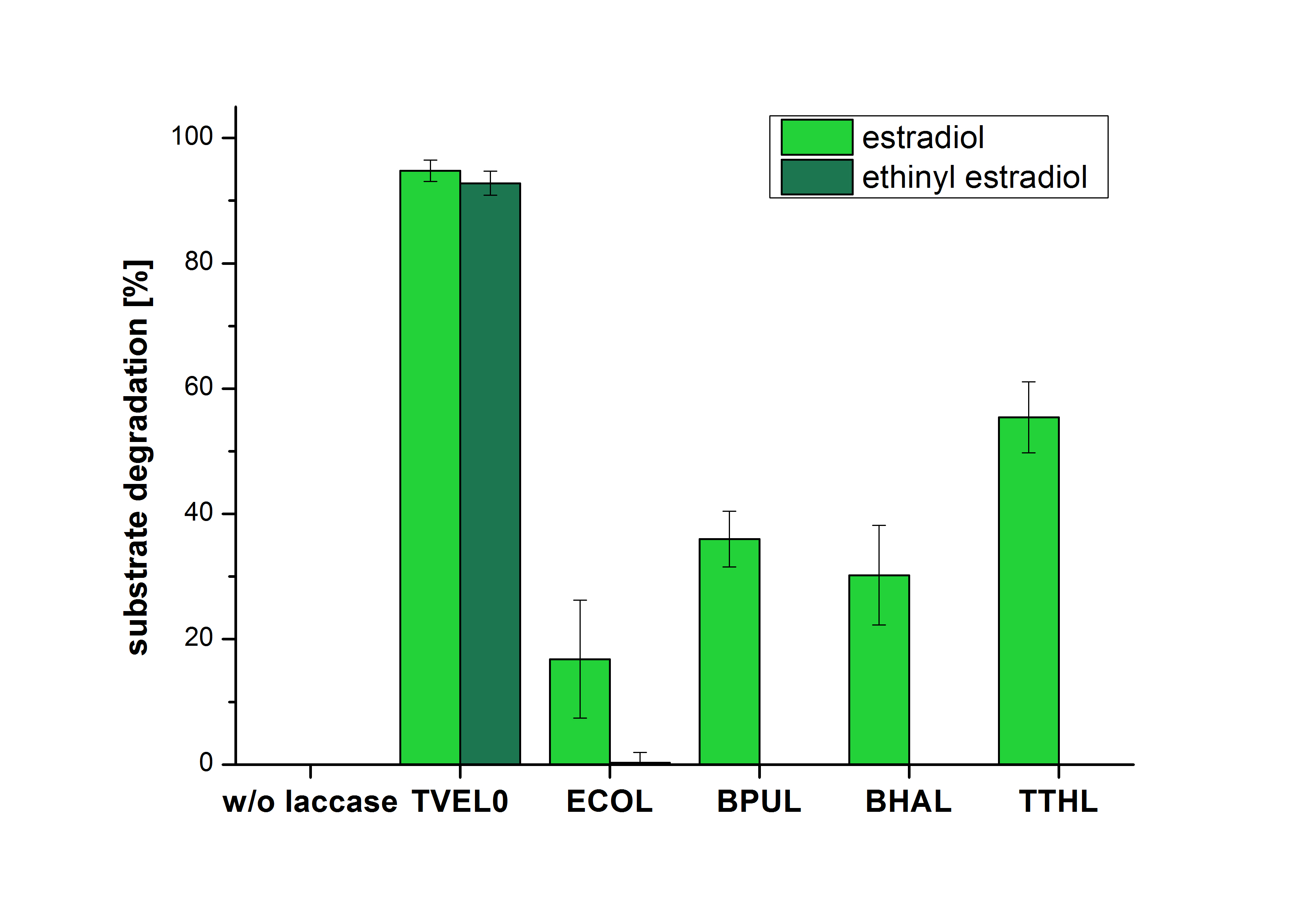
The measurements were made to test if the produced laccases were able to degrade different hormones. Therefore the produced laccases were inserted in the same concentrations (3 µg mL-1) to the different measurement approaches. To work with the correct pH value (which were measured by the Team Activity Test) Britton Robinson buffer at pH 5 was used for all measurements. The initial substrate concentration was 5 µg mL-1. The results of the reactions without ABTS are shown in Figure 17. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are indicated. The X-axis displays the different tested laccases. The degradation was measured at t0 and after five hours of incubation at 30 °C. The negative control was the substrate in Britton Robinson buffer and showed no degradation of the substrates. The bought laccase TVEL0 which is used as positive control is able to degrade 94.7 % estradiol and 92.7 % ethinyl estradiol. The laccase BPUL (from Bacillus pumilus) degraded 35.9 % of used estradiol after five hours. ECOL was able to degrade 16.8 % estradiol. BHAL degraded 30.2 % estradiol. The best results were determined with TTHL (laccase from Thermus thermophilus). Here the percentage of degradation amounted 55.4 %.
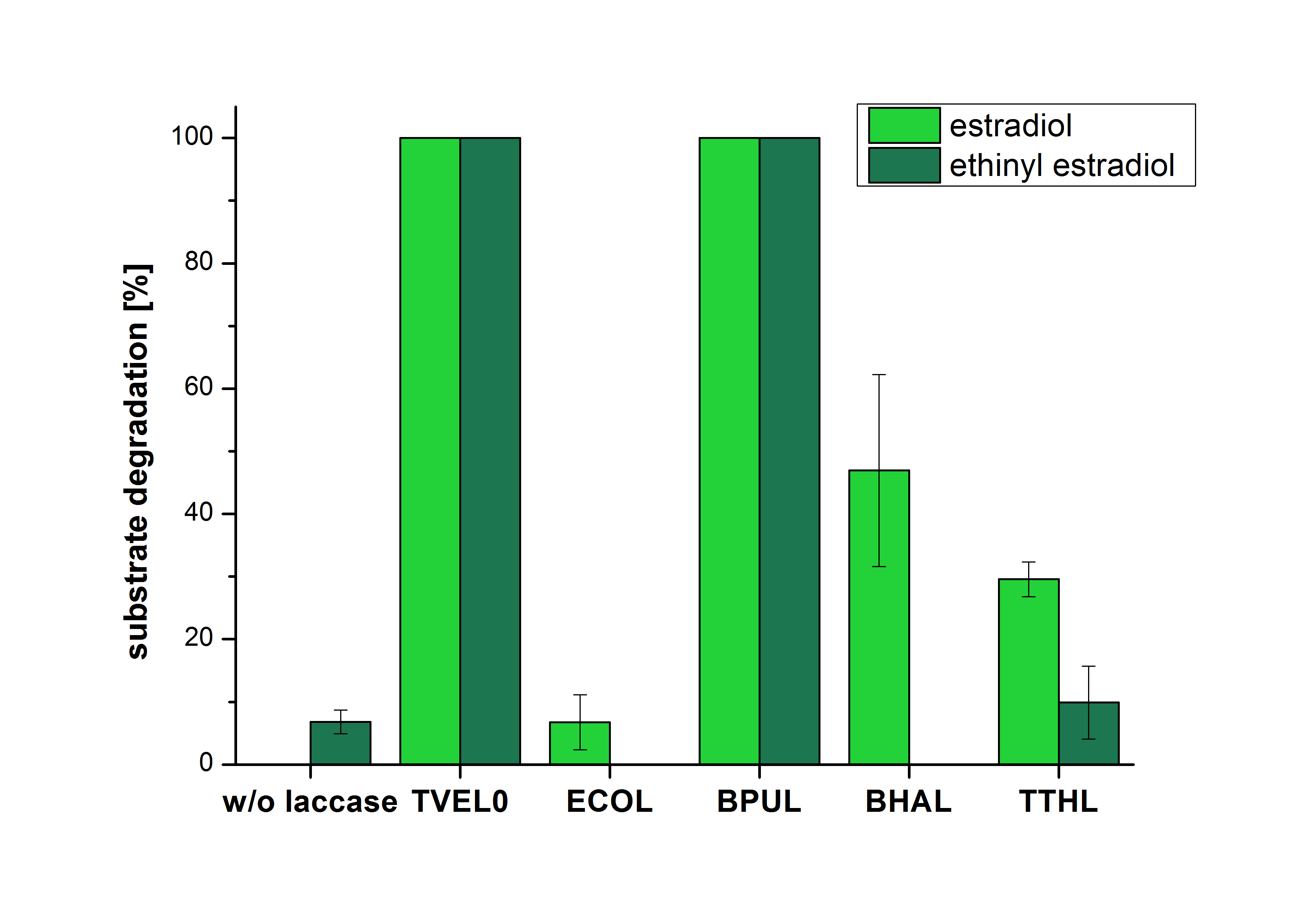
The results of the reactions of the laccases with addition of ABTS are shown in Figure 18. The experimental set ups were the same as the reaction approach without ABTS described above. The X-axis displays the different tested laccases. On the Y-axis the percentages of degraded estradiol (light green) and ethinyl estradiol (dark green) are shown. The degradation was measured at t0 and after five hours of incubation at 20 °C. The negative control showed no degradation of estradiol. 6.8 % of ethinyl estradiol was decayed. The positive control TVEL0 is able to degrade 100 % estradiol and ethinyl estradiol. The laccase BPUL (from Bacillus pumilus) degraded 46.9 % of used estradiol after ten minutes incubation. ECOL was able to degrade 6.7 % estradiol. BHAL degraded 46.9 % estradiol. With TTHL (laccase from Thermus thermophilus) a degradation 29.5 % were determined.
| 55px | | | | | | | | | | |
/partinfo>. The maximal OD /div> ===Since Regionals: Purification of TTHL since Regionals===
 "
"