|
|
| (51 intermediate revisions not shown) |
| Line 19: |
Line 19: |
| | }); | | }); |
| | </script> | | </script> |
| - |
| |
| | | | |
| | <!--this is the top menu--> | | <!--this is the top menu--> |
| Line 79: |
Line 78: |
| | </div> | | </div> |
| | <div id="welcome"> | | <div id="welcome"> |
| - | <P>Future Directions</P> | + | <P>FUTURE WORK</P> |
| | </div> | | </div> |
| | <div id="centercontent"> | | <div id="centercontent"> |
| Line 86: |
Line 85: |
| | <div class="menuword"> | | <div class="menuword"> |
| | <ul> | | <ul> |
| - | <li><a href="#1">Phage's damage towards the fermentation</a></li> | + | <li><a href="#1">Future Directions</a></li> |
| - | <li><a href="#2">Introduction to lambda phage</a></li>
| + | |
| | <ol> | | <ol> |
| - | <li><a href="#2.1"> Genome structure of the toggle switch in lambda phage</a></li> | + | <li><a href="#2.1"> Plasmid Keeping System</a></li> |
| - | <li><a href="#2.2"> Function overview of the proteins involve in the toggle switch</a></li> | + | <li><a href="#2.2"> Quorum Sensing</a></li> |
| - | <li><a href="#2.3"> Maintenance of lysogeny</a></li> | + | <li><a href="#2.3"> Application To Fermentation Industry</a></li> |
| - | <li><a href="#2.4"> Transformation into Lytic life cycle</a></li> | + | <li><a href="#2.4"> Cure For Viral Diseases</a></li> |
| - | <li><a href="#2.4"> Lytic or lysogenic?</a></li>
| + | |
| | </ol> | | </ol> |
| | </ul></div></div> | | </ul></div></div> |
| | + | <br/> |
| | + | <br/> |
| | + | <br/> |
| | + | <br/> |
| | + | <br/> |
| | <br/> | | <br/> |
| | <br/> | | <br/> |
| Line 104: |
Line 106: |
| | <div class="stage"> | | <div class="stage"> |
| | | | |
| - | <h2><a name="1">Phage’s damage towards the fermentation</a></h2> | + | <h2><a name="1">Future Directions</a></h2> |
| - | <div class="imgholder1" align="left" style="float:right;width:320px;height:auto;"> | + | <p>Though the iGEM 2012 is coming to an end,we plan to do further research to make our research accomplished and endue it with new functions. In the future, with enough time and abundant knowledge, these meaningful designs will be realized.</p> |
| - | <a href="https://static.igem.org/mediawiki/2012/5/5a/320px-Tevenphage.png"><img src="https://static.igem.org/mediawiki/2012/5/5a/320px-Tevenphage.png" alt="project image" style="clear:both;width:320;height:229px;"></a><br><small align="center">Diagram of a typical tailed bacteriophage structure</small> | + | <br> |
| | + | |
| | + | <h2><a name="2.1">1.Plasmid Keeping System</a></h3> |
| | + | <div class="imgholder1" align="left" style="float:left;width:289px;height:auto;margin-right:80px;margin-left:50px;"> |
| | + | <a href="https://static.igem.org/mediawiki/2012/3/31/Mechanism_of_the_hoksok_system_in_bacteria_in_the_presence_of_R1_plasmid_DNA.gif"><img src="https://static.igem.org/mediawiki/2012/3/31/Mechanism_of_the_hoksok_system_in_bacteria_in_the_presence_of_R1_plasmid_DNA.gif" alt="Mechanism_of_the_hoksok_system_in_bacteria_in_the_presence_of_R1_plasmid_DNA" style="clear:both;width:289px;height:254px;"></a><br><small align="center">Mechanism of the hok/sok system in bacteria in the presence of R1 plasmid DNA</small> |
| | </div> | | </div> |
| - | <p>The phage is a kind of virus that infect bacteria. Nowadays, nearly each kind of prokaryote has been found to have its specific phage. Generally, a phage contains a head a collar and a tail. The head, namely a protein capsid, contains the phage's genetic material and the tail functions as an anchor for binding on the membrane of the bacteria. The phage is tiny and can only be seen under the EMS (electron microscope). The phage has intense infectivity. What's worse, the phage's resistance against the physical and chemical factors is far too stronger than that of bacteria. It can easily defend the toxic organic solvent such as diethyl ether, the chloroform and the severe circumstance such as low temperature and freeze. However, the phage is sensitive to ultraviolet rays and heat.</p> | + | <div class="imgholder1" align="left" style="float:left;width:289px;height:auto;clear:right;margin-right:30px;"> |
| | + | <a href="https://static.igem.org/mediawiki/2012/9/99/Mechanism_of_the_hoksok_system_in_bacteria_in_the_absence_of_R1_plasmid_DNA.gif"><img src="https://static.igem.org/mediawiki/2012/9/99/Mechanism_of_the_hoksok_system_in_bacteria_in_the_absence_of_R1_plasmid_DNA.gif" alt="Mechanism_of_the_hoksok_system_in_bacteria_in_the_absence_of_R1_plasmid_DNA" style="clear:both;width:289px;height:254px;"></a><br><small align="center">Mechanism of the hok/sok system in bacteria in the absence of R1 plasmid DNA</small> |
| | + | </div> |
| | + | <p>We plan to construct a plasmid keeping system to avoid plasmid loss. In phage-free situations, the bacteria is likely to lose the anti-phage plasmid, therefore a plasmid keeping system is necessary. We choose the hok/sok system to achieve this. It belongs to type I toxin-antitoxin system. The cell will die if the cell lost the plasmid containing the system. Thus, only those cells contain survives. Besides, the type I toxin-antitoxin system is an RNA based system, therefore it won't spend too much energy.</p> |
| | + | <br> |
| | | | |
| - | <p>The phage widely lives in the nature, especially in the air around the manufacturing shops of the fermentation factory and the drainage system. The phage can spread by air and can sneak into nearly every procedure of the fermentation. Main reasons for the pollution of one single fermentation tank contains: remaining blind sides both in the tank and its assistant pipes and the misoperation during inoculating. Main sources of the mass invasion of phages including air filtration system, importation of new strains, lysogen and unthoroughly autoclaved media. All these reasons and sources can lead to continuous pollution among tanks and massive infection.</p> | + | <h2><a name="2.2">2.Quorum Sensing</a></h3> |
| | + | <div class="imgholder1" align="left" style="float:left;width:289px;height:auto;clear:right;margin-bottom:53px;"> |
| | + | <a href="https://static.igem.org/mediawiki/2012/5/52/LamB.jpg"><img src="https://static.igem.org/mediawiki/2012/5/52/LamB.jpg" alt="LamB" style="clear:both;width:289px;height:254px;"></a><br><small align="center">Structure of sugar translocation, also called LamB, the receptor site for lambda phage</small> |
| | + | </div> |
| | + | <div class="imgholder1" align="left" style="float:left;width:289px;height:auto;clear:both;margin-left:60px;"> |
| | + | <a href="https://static.igem.org/mediawiki/2012/b/bd/Aptamer%26LamB.jpg"><img src="https://static.igem.org/mediawiki/2012/b/bd/Aptamer%26LamB.jpg" alt="Ideal function of the aptamer in our future work" style="clear:both;width:289px;height:254px;"></a><br><small align="center">Ideal function of the aptamer in our future work</small> |
| | + | </div> |
| | + | <p>Actually, in the primary plan of our project, our design was that when the suicide gene works, the host would use quorum sensing to alarm the bacteria around and make them prepare for the defense. We don't hope the bacteria spend a lot of energy on the defense when no phage invades. To this end, we design that the defense action will be taken only when the bacteria receive the quorum sensing signal, which is sent by the host. The reason for giving up this feature is that we haven't found any effective defense method. The defense method in our plan was blocking the phage’s binding site on the membrane of bacteria. We learned that a sugar transporter called LamB (the structure is showed below) may be the phage’s binding site and we planned to use aptamer to block this site. </p> |
| | + | <div class="imgholder1" align="left" style="float:left;width:319px;height:auto;clear:right;margin-right:40px;margin-left:50px;"> |
| | + | <a href="https://static.igem.org/mediawiki/2012/e/ed/LamB_on_membrane_%E5%89%AF%E6%9C%AC.jpg"><img src="https://static.igem.org/mediawiki/2012/e/ed/LamB_on_membrane_%E5%89%AF%E6%9C%AC.jpg" alt="Proposed model for the folding of LamB in the outer membrane." style="clear:both;width:319px;height:254px;"></a><br><small align="center">Proposed model for the folding of LamB in the outer membrane.</small> |
| | + | </div> |
| | | | |
| - | <p>In fermentation production, the loss results from the bacteria's being infected by the phage is striking. Because the phage infect intensely and spread rapidly, once the bacteria are infected, it is very difficult to eradicate the phages and the lysogens thoroughly. For this reason, it is quite important to prevent the invasion of the phages. If large tanks of fermentation broth are only polluted slightly, the period of the fermentation will be extended, the broth will become clear gradually and the synthesis of the fermented products will be much more difficult. However, if the fermentation broth is unluckily polluted severely, the factory will have to stop production totally, and massive loss in profit happens afterward. This kind of catastrophe occurs commonly in the fermentation of glutamic acid, amylase, protease, acetone and many kinds of antibiotics. For example, in the fermentation of lactic acid, the seed culture of the lactobacillus will stop production in 30 minutes once the bacteria are infected severely by phages.</p> | + | <p>But unfortunately, there are too many difficulties we have to face. For example, we haven't find a method to make the aptamer get through the inner membrane of E.coli. What’s more, LamB is so complex that it will be quite difficult for us to get an aptamer that works.(see the proposed model for the folding of LamB in the outer membrane.).</p> |
| | | | |
| - | <p>Some common phenomena that will occur if the fermentation broth is polluted by the phages are listed below:</p> | + | <p>Therefore in the future,we will raise some new ideas complete the quorum sensing part.</p> |
| - | <li>1. The bacteria will die and lyse in a short time and only the fragments of bacteria will remain afterward.</li>
| + | |
| - | <li>2. OD at 600nm of the fermentation broth can be found quite low.</li>
| + | |
| - | <li>3. Under the microscope, you can see the number of the bacteria is extremely decreased and the bacteria in a colony are abnormally arranged. What's worse, there are no complete bacteria can be found.</li>
| + | |
| - | <li>4. The physicochemical properties are changing. The pH is increasing, the temperature stop climbing and start to decrease gradually. The amount of CO2 emitted is dropping rapidly.</li>
| + | |
| - | <li>5. The metabolism of the bacteria becomes abnormal and the production of the fermentation products stops.</li>
| + | |
| - | <li>6. Plenty of bubbles emerge and the broth appears red and grey. Sometimes the broth can be even sticky.</li>
| + | |
| - | <li>7. Plaques can be seen when using double-layer media to test the bacteria.</li>
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| | | | |
| | | | |
| - | <h2><a name="2">Introduction to lambda phage</a></h2> | + | <h2><a name="2.3">3.Application To Fermentation Industry</a></h3> |
| - | <p>Entcrobacteria phage λ (lambda phage, coliphage λ) is a bacterial virus, or bacteriophage, that infects the bacterial species Escherichia coli. This virus is temperate and may reside within the genome of its host through lysogeny or amplify its number and lyse the host through lytic life cycle.</p> | + | <p>We plan to apply this method to other fermentation industries. In general, our circuit in the engineered bacteria will work if we replace the promoter pRM with a specific one. We will also select a suicide gene which is biocompatible with the target and can also promote the quorum sensing.</p> |
| - | <div class="imgholder1" align="left" style="float:right;width:200px;height:auto;">
| + | |
| - | <a href="https://static.igem.org/mediawiki/2012/2/25/593px-LambdaPlaques.jpg"><img src="https://static.igem.org/mediawiki/2012/2/25/593px-LambdaPlaques.jpg" alt="project image" style="clear:both;width:200px;height:200px;"></a><br><small align="center">Lysis plaques of lambda phage on E. coli bacteria lambda phage</small>
| + | |
| - | </div>
| + | |
| - | <p>Usually, a <a href="">"lytic cycle"</a> ensues, where the lambda DNA is replicated many times and the genes for head, tail and lysis proteins are expressed. This leads to assembly of multiple new phage particles within the cell and subsequent cell lysis, releasing the cell contents, including virions that have been assembled, into the environment</p>
| + | |
| | | | |
| - | <p>However, under certain conditions the phage DNA may integrate itself into the host cell chromosome in the <a href="">lysogenic pathway</a>. In this state, the λ DNA is called a prophage and stays resident within the host's genome without apparent harm to the host. The host can be termed a lysogen when a prophage is present.</p><br>
| |
| - | <h3><a name="2.1">Genome structure of the toggle switch in lambda phage</a></h3>
| |
| - | <div class="imgholder1" align="left" style="clear:both;width:289px;height:auto;margin-left:100px;">
| |
| - | <a href="https://static.igem.org/mediawiki/2012/6/6f/Bacteriophage_lambda_genome_total.jpg"><img src="https://static.igem.org/mediawiki/2012/6/6f/Bacteriophage_lambda_genome_total.jpg" alt="Bacteriophage_lambda_genome_total" style="clear:both;width:289px;height:254px;"></a><br><small align="center">Schematic representation of the genome of the bacteriophage lambda.</small>
| |
| - | </div>
| |
| - | <div class="imgholder1" align="left" style="float:right;width:235px;height:auto;">
| |
| - | <a href="https://static.igem.org/mediawiki/2012/f/f7/Sequence_of_toggle_switch_%E5%89%AF%E6%9C%AC.jpg"><img src="https://static.igem.org/mediawiki/2012/f/f7/Sequence_of_toggle_switch_%E5%89%AF%E6%9C%AC.jpg" alt="project image" style="clear:both;width:235px;height:109px;"></a><br><small align="center">Sequence_of_toggle_switch</small>
| |
| - | </div>
| |
| - | <p><b>Promoter pR:</b>>Initializes the rightward transcription. If the pR is not repressed, these proteins will be expressed and the phage will transfer into lytic cycle and plenty of new phage particles will be assembled.</p>
| |
| - | <p><b>Promoter pRM:</b>Initializes the transcription of repressor CI. It transcripts leftwards.
| |
| - | OR1, OR2, OR3: Three protein binding regions on the genome of lambda phage. They can be bound by protein CI dimmers(CI2) and Cro dimmers(Cro2). The sites of these three regions on DNA are shown in the picture below.</p>
| |
| | <br> | | <br> |
| - | <h3><a name="2.2">Function overview of the proteins involve in the toggle switch</a></h3>
| |
| - | <p><span>Cro:<span> Transcription inhibitor. The protein Cro can automatically form diamer Cro2 and then binds OR3, OR2 and OR1 (affinity OR3 > OR2 = OR1, i.e. preferentially binds OR3) (<a href="">See the free energy of CI2 or Cro2 interacting with the three regions</a>)..At low concentrations the Cro2 blocks the pRM promoter, preventing cI production afterwards. At high concentrations the Cro2 downregulates its own production through OR2 and OR1 binding. No cooperative binding (c.f. below for cI binding)</p>
| |
| - | <p><span>CI:<span> Transcription inhibitor. The protein CI can also automatically form diamer CI2(similar to Cro2) and then binds OR1, OR2 and OR3 (affinity OR1 > OR2 = OR3, i.e. preferentially binds OR1). At low concentrations the CI2 blocks the pR promoter, preventing cro production afterwards. At high concentrations the CI2 downregulates its own production through OR3 binding. N terminal domain of cI on OR2 tightens the binding of RNA polymerase holoenzyme complex to pRM and hence stimulate its own transcription. Repressor also inhibits transcription from the pL promoter.</p>
| |
| - | <h3><a name="2.3">Maintenance of lysogeny</a></h3>
| |
| - | <p>Lysogeny is maintained solely by cI. cI represses transcription from pL and pR while upregulating and controlling its own expression from pRM. It is therefore the only protein expressed by lysogenic phage.</p>
| |
| | | | |
| - | <div class="imgholder1" align="right" style="float:right;width:800px;height:auto;margin-right:0px;">
| + | |
| - | <a href="https://static.igem.org/mediawiki/2012/0/02/ThebacteriophagelCIproteinfindsanasymmetricsolution%28b%29.jpg"><img src="https://static.igem.org/mediawiki/2012/0/02/ThebacteriophagelCIproteinfindsanasymmetricsolution%28b%29.jpg" alt="project image" style="clear:both;width:800px;height:267px;"></a><br><p><small align="center">CI dimers bound cooperatively to adjacent operator sites in OR and OL. The CI dimers are shown in blue. Each subunit of the dimer consists of an N-terminal DNA-binding domain (N), a C-terminal oligomerization domain(C), and a linker region (black) connecting the two. The dimer pair bound cooperatively at OR1 and OR2 represses transcription from PR and the dimer bound at OR2 also activates transcription from PRM. The dimer pair bound cooperatively at OL1 and OL2 represses transcription from PL.</small></p>
| + | |
| - | </div> | + | <h2><a name="2.4">4.Cure For Viral Diseases</a></h3> |
| - | <h3><a name="2.4">Transformation into Lytic life cycle</a></h3>
| + | <p>If possible, we will make some medical research with this method, because this anti-virus method is universal. It will be a possible way on therapies against viral diseases, such as the HIV and the Flu.</p> |
| - | <p>Cro is responsible for preventing the synthesis of the repressor CI and this action shuts off the possibility of establishing lysogeny. It has two effects:</p> | + | |
| - | <ul>
| + | |
| - | <li>It prevents the synthesis of repressor via the maintenance circuit; that is, it prevents transcription via pRM .</li>
| + | |
| - | <li>It also inhibits the expression of early genes from both pL and pR
| + | |
| - | Cro achieves its function by binding to the same operators as (el) repressor protein. </li>
| + | |
| - | </ul>
| + | |
| | <br/> | | <br/> |
| - | <h3><a name="2.5">Lytic or lysogenic? </a></h3>
| + | |
| - | <p>The gene regulatory circuitry of phage λ is among the best-understood circuits at the mechanistic level. This circuitry involves several interesting regulatory behaviors. An infected cell undergoes a decision between two alternative pathways, the lytic and lysogenic pathways, just like two states controlled by a toggle switch.</p>
| + | |
| - | <p>Simplistically, in cells with sufficient nutrients, protease activity is high, which breaks down cI.[10] This leads to the lytic lifestyle. In cells with limited nutrients, protease activity is low, making cI stable. This leads to the lysogenic lifestyle. This means that a cell "in trouble", i.e. lacking in nutrients and in a more dormant state, is more likely to lysogenise. This would be selected for because the phage can now lie dormant in the bacterium until it falls on better times, and so the phage can create more copies of itself with the additional resources available and with the more likely proximity of further infectable cells.</p>
| + | |
| | <br/> | | <br/> |
| | <br/> | | <br/> |
| Line 189: |
Line 172: |
| | <p class="level1"><a href="https://2012.igem.org/Team:USTC-China/methods">Methods</a></p> | | <p class="level1"><a href="https://2012.igem.org/Team:USTC-China/methods">Methods</a></p> |
| | | | |
| - | <p class="level0"><a href="https://2012.igem.org/Team:USTC-China/achievements">ACHIEVEMENTS</a></p> | + | <p class="level0"><a href="https://2012.igem.org/Team:USTC-China/results">ACHIEVEMENTS</a></p> |
| | | | |
| | <p class="level1"><a href="https://2012.igem.org/Team:USTC-China/results">Results</a></p> | | <p class="level1"><a href="https://2012.igem.org/Team:USTC-China/results">Results</a></p> |
Though the iGEM 2012 is coming to an end,we plan to do further research to make our research accomplished and endue it with new functions. In the future, with enough time and abundant knowledge, these meaningful designs will be realized.
1.Plasmid Keeping System
 Mechanism of the hok/sok system in bacteria in the presence of R1 plasmid DNA
Mechanism of the hok/sok system in bacteria in the presence of R1 plasmid DNA
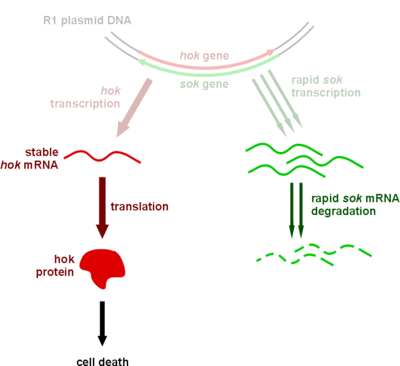 Mechanism of the hok/sok system in bacteria in the absence of R1 plasmid DNA
Mechanism of the hok/sok system in bacteria in the absence of R1 plasmid DNA
We plan to construct a plasmid keeping system to avoid plasmid loss. In phage-free situations, the bacteria is likely to lose the anti-phage plasmid, therefore a plasmid keeping system is necessary. We choose the hok/sok system to achieve this. It belongs to type I toxin-antitoxin system. The cell will die if the cell lost the plasmid containing the system. Thus, only those cells contain survives. Besides, the type I toxin-antitoxin system is an RNA based system, therefore it won't spend too much energy.
2.Quorum Sensing
 Structure of sugar translocation, also called LamB, the receptor site for lambda phage
Structure of sugar translocation, also called LamB, the receptor site for lambda phage
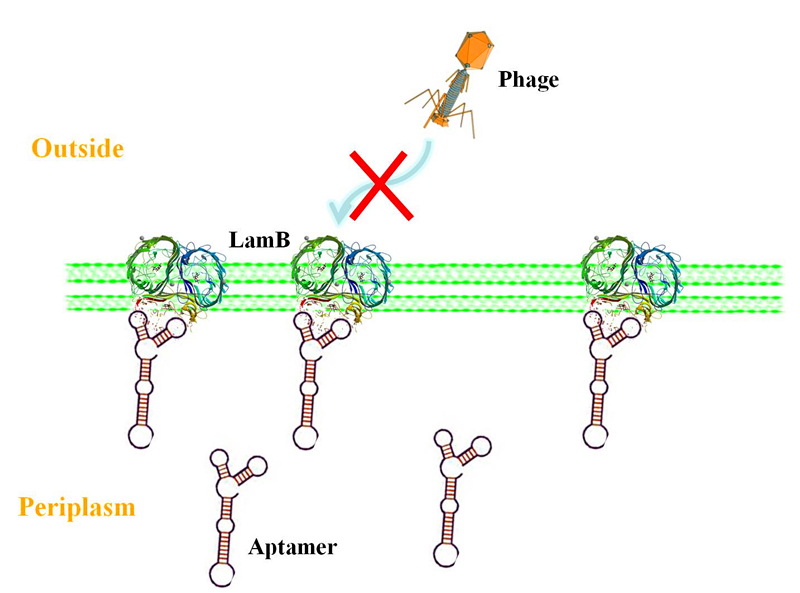 Ideal function of the aptamer in our future work
Ideal function of the aptamer in our future work
Actually, in the primary plan of our project, our design was that when the suicide gene works, the host would use quorum sensing to alarm the bacteria around and make them prepare for the defense. We don't hope the bacteria spend a lot of energy on the defense when no phage invades. To this end, we design that the defense action will be taken only when the bacteria receive the quorum sensing signal, which is sent by the host. The reason for giving up this feature is that we haven't found any effective defense method. The defense method in our plan was blocking the phage’s binding site on the membrane of bacteria. We learned that a sugar transporter called LamB (the structure is showed below) may be the phage’s binding site and we planned to use aptamer to block this site.
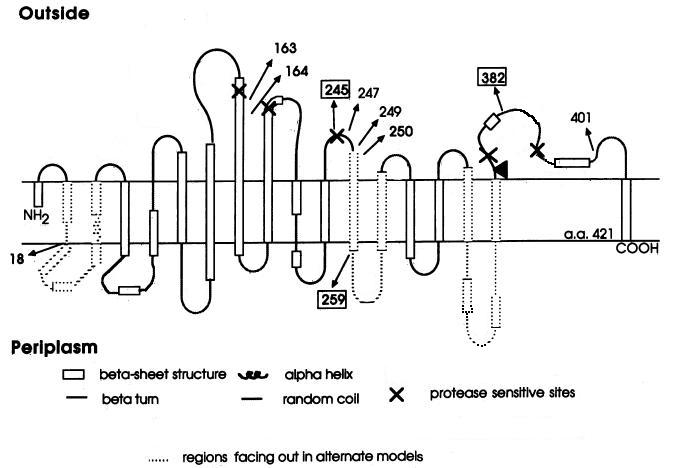 Proposed model for the folding of LamB in the outer membrane.
Proposed model for the folding of LamB in the outer membrane.
But unfortunately, there are too many difficulties we have to face. For example, we haven't find a method to make the aptamer get through the inner membrane of E.coli. What’s more, LamB is so complex that it will be quite difficult for us to get an aptamer that works.(see the proposed model for the folding of LamB in the outer membrane.).
Therefore in the future,we will raise some new ideas complete the quorum sensing part.
We plan to apply this method to other fermentation industries. In general, our circuit in the engineered bacteria will work if we replace the promoter pRM with a specific one. We will also select a suicide gene which is biocompatible with the target and can also promote the quorum sensing.
If possible, we will make some medical research with this method, because this anti-virus method is universal. It will be a possible way on therapies against viral diseases, such as the HIV and the Flu.
 "
"






