Team:Buenos Aires/Results/BBsTesting
From 2012.igem.org

Contents |
After the Jamboree!
When we returned from the LatinAmerican Jamboree we focused all our effords on completing the neccesary transformations to test our devices and our projet as a whole.
We devided this task in three sections
Week 1&2 : Yeast expression vectors & Transformations
| YFP_TRP+++_TRPZipper | details | |
| YFP_TRP+_TRPZipper | details | |
| YFP_TRP+++_PoliWb | details | |
| YFP_TRP+_PoliWb | details | |
| CFP_HIS+_PoliHa | details | |
| CFP_HIS+_PoliHb | details |
Week 3: synthetic Ecology Characterization
xxoo
BB characterization
xxoo
Secretion Rate of Trp as a function of culture growth
The aim of this experiment is to characterize Trp secretion on devices 2 and 4. We cultured the transformed strains overnight in 5 ml of medium +H-T until they reached an OD: 0.1 (exponential phase).
In order to have a clear signal we used:
TCY3081 <- pCM185 <- Device 2
TCY3081 <- pCM185 <- Device 4
TCY3081 <- pCM185
TCY3081 <- pCM182 <- Device 2
TCY3081 <- pCM182 <- Device 4
TCY3081 <- pCM182
TCY3081
We did 3 replica of each culture. As from this point we measured OD every hour until they reached an OD: 0.8 (approximately). We then measured the Trp signal for each culture medium using the spectrofluorometer.
Trp Secretion at different His concentrations in medium and His secretion at different Trp concentrations in medium
We started cultures in -T of the following strains:
i. strain TCY3081+pCM185 containing device2 ii. strain TCY3081+pCM185 containing device4 iii. strain TCY3081+pCM185 (empty plasmid)
We measured the OD of the cultures after 12 hs. We prepared cultures with increasing concentrations of His in medium (1X, 1/4; 1/8; 0X)and set cultures at initial OD: 0.1. After 5 hours we measured the final OD and Trp in medium.
Strain characterization
Experimental determination of K death
In order to determinate the K death used in the modeling section of our wiki, we set cultures of the two auxotrophic strains without being transformed (TCY3081 and TCY3128) in medium –HT at an initial OD of 0.01.
Taking into account that an OD:1 is approximately 3.10 7 cells/ml, we plated 133 µl in order to have around 200 colonies in the first day. Each following day we plated the same amount of µl of the culture and counted the number of colonies obtain in each plate. We set 3 replica of each strain.
TABLA CON EL NUMERO DE CELULAS POR DIA
Growth rate vs [TRP] and [HIS]
We made dilutions in series for increasing concentrations of Trp or His(1/32; 1/16; 1/8 ; 1/4; 1/2; 1X) in a -T or -H medium respectively. We made 2 replica of each concentration.
We set the cultures of the different strains at a low initial OD:0.001 approximately.
i. strain TCY3081 without device in each Trp concentration
ii. strain TCY3128 without device in each His concentration
iii. strain TCY3081 without device in Synthetic Complete medium
iv. strain TCY3128 without device in Synthetic Complete medium
v. Control (Synthetic Complete medium)
We measured OD reached by cultures 12 hs after started.
CONVERTIR ESTO EN UNA TABLA CON LOS RESULTADOS
Co-Culture of strains
We proceeded then to test the coculture growth of transformed strains with devices vs transformed strains with empty devices and non transformed strains.
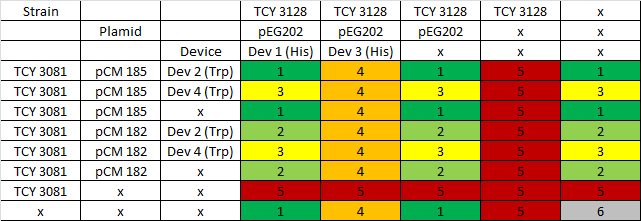
|
Table: Coculture planification. Number indicates level of priority of the experiment for characterization of devices.
Due to a time constrain, we ordered the experiments by level of priority and proceeded to test our devices by cocultures 1 and 2.
Starters of each strain were done in medium complementary to the auxotrophy of the strain so that they would maintain their plamids (medium –H for pEG202; medium –T for pCM182/5 and medium SC for cells without plasmid), then sonicated briefly in low power and then washed with medium –H-T . When they are in exponential phase, we set the coculture of strains was at OD 600: 0.02 (Total, 1:1), in 5 ml of medium –H-T.
We used epifluorescence microscope in order to determinate the strain proportion of each coculture. We used a 384 wells plate , with 20 µl of cyclohexamide 2x (CHX 2x) in each of the wells where we placed a sample. The density of the culture was calculated based on the cell density at in each wells.
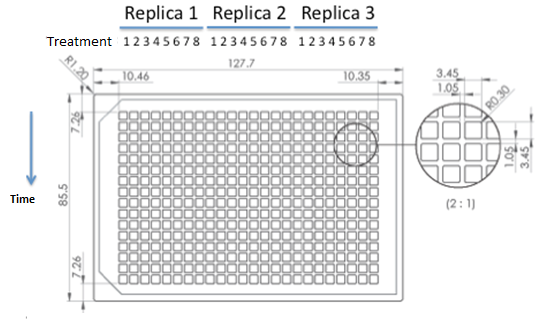
|
Graph: 384 wells plate to be used for epifluorescence microscope.
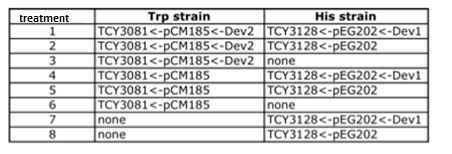
|
Table: Coculture planification for number 1.
 "
"