|
|
| Line 8: |
Line 8: |
| | Following our experimental strategy we built two plasmid backbones on which to insert the Lux constructs. | | Following our experimental strategy we built two plasmid backbones on which to insert the Lux constructs. |
| | | | |
| - | <h2>pSB1C3_IntK</h2> | + | <h2>Neutral recombination plasmid</h2> |
| | | | |
| | <div id="boxright"> | | <div id="boxright"> |
| Line 30: |
Line 30: |
| | | | |
| | | | |
| - | <h3>Bacterial luciferase(s)</h3> | + | <h3>Insertion of LuxAB genes</h3> |
| | <br> | | <br> |
| | | | |
| Line 39: |
Line 39: |
| | <div id="boxright"> | | <div id="boxright"> |
| | [[File: LuxABxl_digestion.jpg| 400px| right]] | | [[File: LuxABxl_digestion.jpg| 400px| right]] |
| | + | Lane 1 = Ladder 1Kb, Lane 3 = |
| | </div> | | </div> |
| | | | |
| Line 63: |
Line 64: |
| | <br> | | <br> |
| | | | |
| - | <h2>pSB1C3_IntS</h2> | + | <h2>Suceptibility construct</h2> |
| | | | |
| | <br> | | <br> |
| Line 109: |
Line 110: |
| | | | |
| | | | |
| - | <h3>Substrate production under Pcaa3</h3> | + | <h3>Insertion of Lux substrate biosynthesis genes under twilight promoters</h3> |
| | | | |
| - | We have amplified all the parts of the substrate production pathway ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K325902 LuxCD] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K325903 LuxEG]) for the final construct under the Pcaa3 promoter (which has also been Biobricked with code [[http://partsregistry.org/wiki/index.php?title=Part:BBa_K743002 K743002]. Gibson assembly of the parts will be done after the wiki freeze. | + | We have amplified all the parts of the substrate production pathway ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K325902 LuxCD] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K325903 LuxEG]) for the final construct under the Pcaa3 promoter (which has also been Biobricked with code [[http://partsregistry.org/wiki/index.php?title=Part:BBa_K743002 K743002]. Also, are amplifying one remaining part (LuxCD with 5' homology to PsigE) for doing gibson assembly of the LuxCDEG construct under PsigE. Gibson reaction will be done together with the substrate production construct under Pcaa3 in pSB1C3_IntS for both constructs. |
| - | | + | |
| - | <h3>Substrate production under PsigE</h3>
| + | |
| - | | + | |
| - | We are amplifying one remaining part (LuxCD with homology to PsigE) for doing gibson assembly of the LuxCDEG construct under PsigE. Gibson reaction will be done together with Substrate production constuct under Pcaa3 in pSB1C3_IntS for both constructs.
| + | |
| | | | |
| | <h1>Synechocystis PCC. 6803</h1> | | <h1>Synechocystis PCC. 6803</h1> |

Plasmid Construction
Following our experimental strategy we built two plasmid backbones on which to insert the Lux constructs.
Neutral recombination plasmid
 1st lane is 1Kb Ladder. 4th lane is C4; 1rst band = Digestion intermediate, 2nd band = pSB1C3 backbone, 3rd band = RS1-KanR-RS2partial, 4th band = RS2partial.
1st lane is 1Kb Ladder. 4th lane is C4; 1rst band = Digestion intermediate, 2nd band = pSB1C3 backbone, 3rd band = RS1-KanR-RS2partial, 4th band = RS2partial.
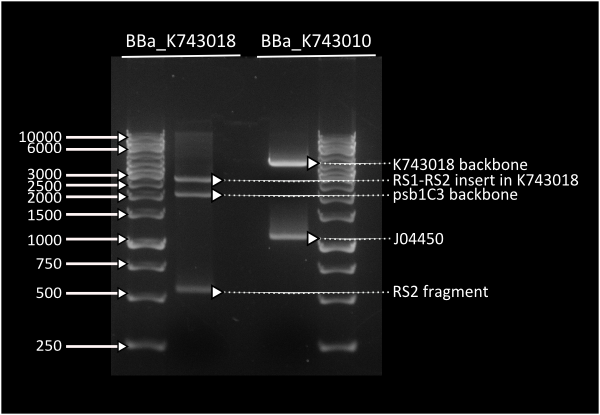
This plasmid backbone was constructed by standard assembly techniques. Recombination sites ([http://partsregistry.org/Part:BBa_K743000 BBa_K743000] and [http://partsregistry.org/Part:BBa_K743001 Part:BBa_K743001]) were Biobricked by gibson assembly, which were consenquently ligated to neighboring parts B0015 and KanR in parallel assembly. Afterwards, we ligated both composites between them through a standard assembly reaction. Final construct was validated by digestion (see gel image at right) and corroborated through sequencing.
After obtaining [http://partsregistry.org/Part:BBa_K743006 BBa_K743006], we proceeded assembling our constructs through Gibson Assemblies.
If you wondering on why the plasmid was constructed by standard assembly and not by gibson assembly from scratch, please refer to Gibson assembly for small parts
Insertion of LuxAB genes
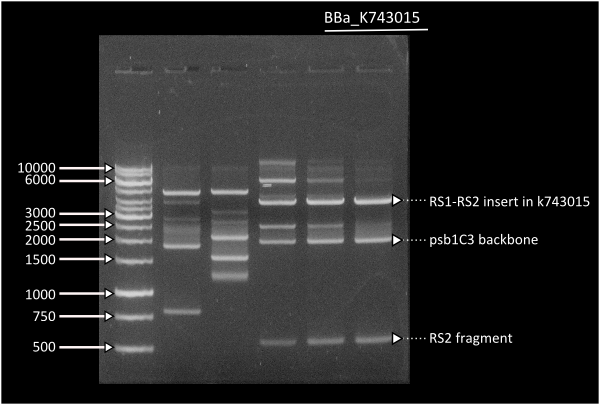
Starting from [http://partsregistry.org/Part:BBa_K743006| K743006] as a plasmid backbone, we were able to build our LuxAB constructs for the bacterial luciferase using 2 different versions available at the registry (from [http://partsregistry.org/Part:BBa_K743014 Photorhabdus luminiscent, BBa_K743014] and [http://partsregistry.org/Part:BBa_K743015 Vibrio fisherii, BBa_K743015]) under an endogenous Synechocystis's promoter (transaldolase Reference???). Resulting constructs were verified by digestion (see gel images below) and corroborated by sequencing.
Lane 1 = Ladder 1Kb, Lane 3 =
Suceptibility construct
pSB1C3_IntS was constructed in two successive gibson assemblies. The first assembly inserted the CopS region (the entire RS5 & RS6 recombination locus) to a chopped-down pSB1C3 lacking the whole VF2 to VR region. The second assembly inserted the Spectynomycin resistance selection marker and the whole VF to VR region of plasmid pSB4K5-J04450 right in between of the RS5 & RS6 CopS region. This construct yields red colonies if correctly assembled (see image at left). Red colonies were colony PCRed, verificated by digestion (see image down right) and corroborated by sequencing.
Insertion of Lux substrate biosynthesis genes under twilight promoters
We have amplified all the parts of the substrate production pathway ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K325902 LuxCD] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K325903 LuxEG]) for the final construct under the Pcaa3 promoter (which has also been Biobricked with code [[http://partsregistry.org/wiki/index.php?title=Part:BBa_K743002 K743002]. Also, are amplifying one remaining part (LuxCD with 5' homology to PsigE) for doing gibson assembly of the LuxCDEG construct under PsigE. Gibson reaction will be done together with the substrate production construct under Pcaa3 in pSB1C3_IntS for both constructs.
Synechocystis PCC. 6803
Once constructs were corroborated by sequencing, we proceeded to transform synechocystis. Prior to transforming, we verified that our Synechocystis cultures were in exponential growth by comparison to our growth curve characterization.
Transforming Synechocystis PCC 6803
(At the right side of the screen a 1 day old transformation plate of Synechocystis PCC. 6803 is shown)
After corroborating our constructs we proceded with the Synechocystis transformation for each of the constructs as soon as we obtained sufficient concentration (10ug) of the construct.
Transformation in Synechocystis takes about 2 weeks to reveal transformant colonies, which in turn have to restreaked again before PCR verification. This makes working in Synechocystis pressing as mistakes can take long to unravel.
LuxABxl under transaldolase promoter transformation
This picture shows the transformation of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K743014 K743014] after 14 days. On the left there is the control transformation plate (no DNA) on Kanamycin 25ug/mL and on the right the succesful transformation of Synechocystis colonies growing througout the Millipore membrane on the same antibiotic.
LuxABvf under transaldolase promoter transformation
It is important to note that to our knowledge we are the first iGEM team to do a succesful direct transformation of Synechocystis PCC. 6803 with naked DNA.
sfGFP with LVA tag for describing circadian behaviour
To describe the circadian behaviour of the promoter we built a fast-degrading reporter consisting of sfGPF I746916 with a LVA degradation tag in the C-terminal end of the protein. This construct will serve as a real-time reporter of promoter activity, you may find more information about the half-life of proteins with the LVA tag [http://partsregistry.org/wiki/index.php?title=Part:BBa_M0050 | here]. The construct has been verified by digestion and corroborated by sequencing.
 "
"