LuxBrick Characterization - (BBa_K325909)
As a part of our project, we set out to investigate the conditions which affect the output of the LuxBrick. As we are using various elements from this Biobrick, we believe that is cornersome to know how we may increase its output by modifying the conditions that affect its behaviour.
We set out to understand diverse factors that could be influencing the total bioluminescence yield of the brick. Some of them have direct relation with specific of the aspects of our project. These factors are:
Glucose concentration
Growth state at time of induction
Cell mass
Temperature
Here you'll find detailed the characterization methods we used in this experiment.
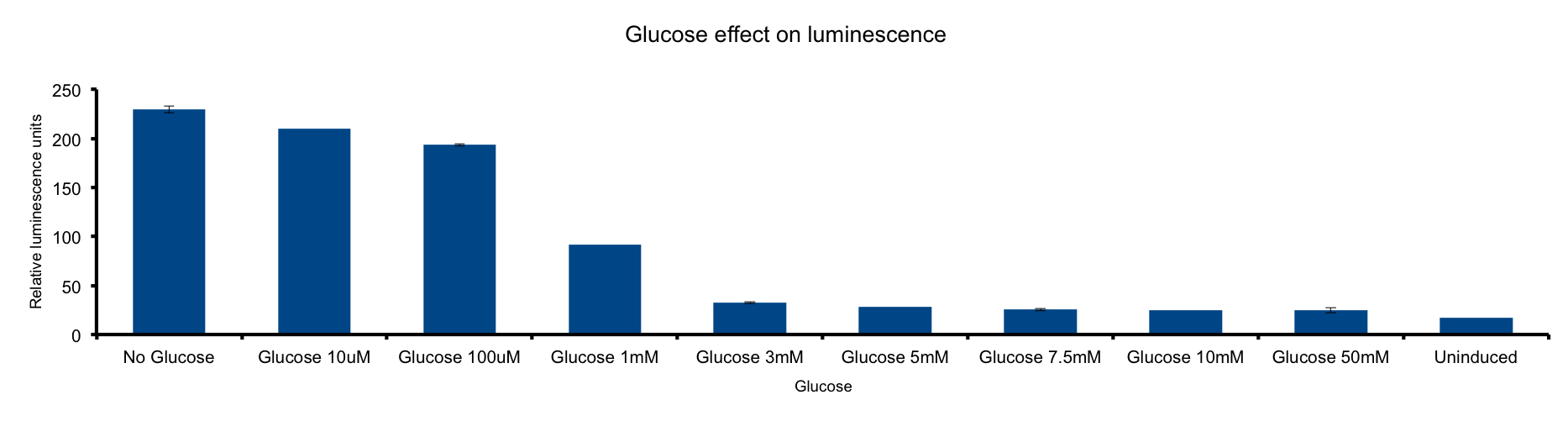
Glucose concentration in media
Glucose is a molecule central for the energy metabolism throughout living organisms and its the main energy source for E.coli.
As reduced carbon-hydrogen bonds in glucose are oxidized through glicolisis and TCA cycle, reduced equivalents are stored in NADPH and FADH2. The majority of these electrons finally react with molecular oxygen to form water at the end of the electron transport chain (ETC), reaction that is catalized by citochrome c oxydase.
This metabolic pathway is linked to the Lux light emition in two ways: the luciferase reaction consumes reducing power and in the other hand it can be considered respiration as it consumes oxygen 1.
Our results clearly show a strong inhibition of the light emition down to non-induced levels at glucose concentrations higher than 3mM . Our hypothesis is that ETC respiration competes for oxygen with luciferase respiration, then, if glucose is added to de medium, the reduction of oxygen to water would be enhanced, making oxygen less available to luciferase.
The fact that LuxAB light emittion is augmented by cytochrome c ocydase inhibition supports this idea 2.
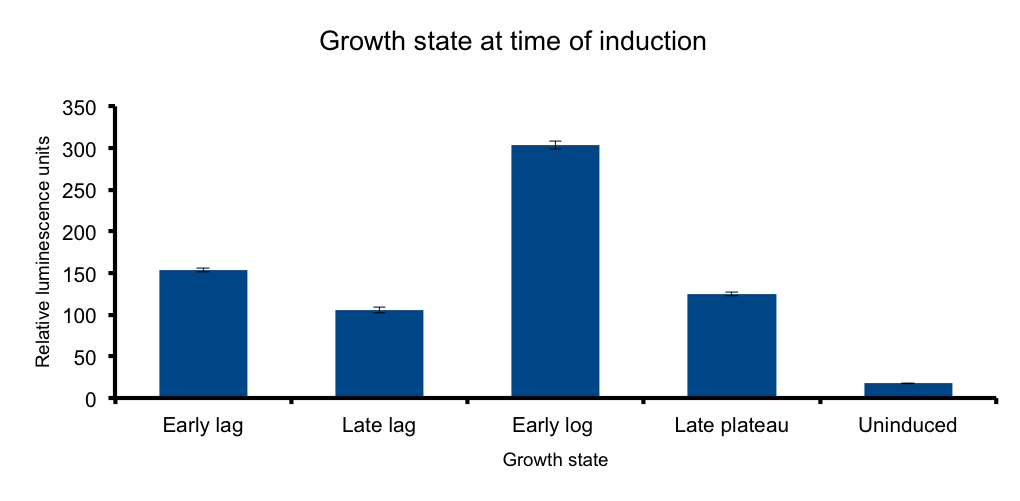
Growth state at-time-of-induction
It has been know for some time that general bacterial metabolism is determined by its growth state 3. Growth phase determines available resources and stress derived restrictions. Bacteria can dramatically adjust their metabolism to adapt to these changing conditions.
At the transcriptional level, different sigma subunits present at different growth states regulate gene expression throughout the whole genome(ref3)
We hipotezised that an heterologous metabolic pathway such as Lux light emittion wouldn´t be free from this regulation.
Our results confirm our hipotesis: measured luminiscence output is growth state dependent, low at the early lag phase, peaking at early log phase and decaying at late stationary phase.
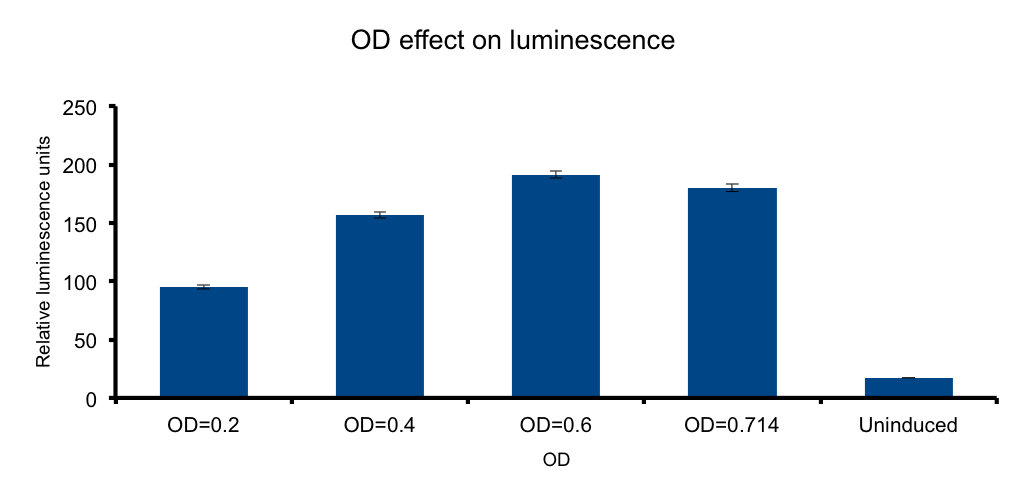
Cell mass (Optical Density)
If cells dilluted in a liquid medium behave as light emitting entities, the light transmitted to the exterior will depend on the cell density. We wanted to know if there is an optimal density, under which there are not enough cells to achieve maximum emition and over which the very same cells will absorb the emited photons therefore diminishing the emited light because of the turbidity
Our measurments showed that such an optimal o.d it´s not consistent with the results. The model that best adjusts to our data is an asymptotic effect of o.d over luminiscence, reaching an maximum at o.d 0,6.
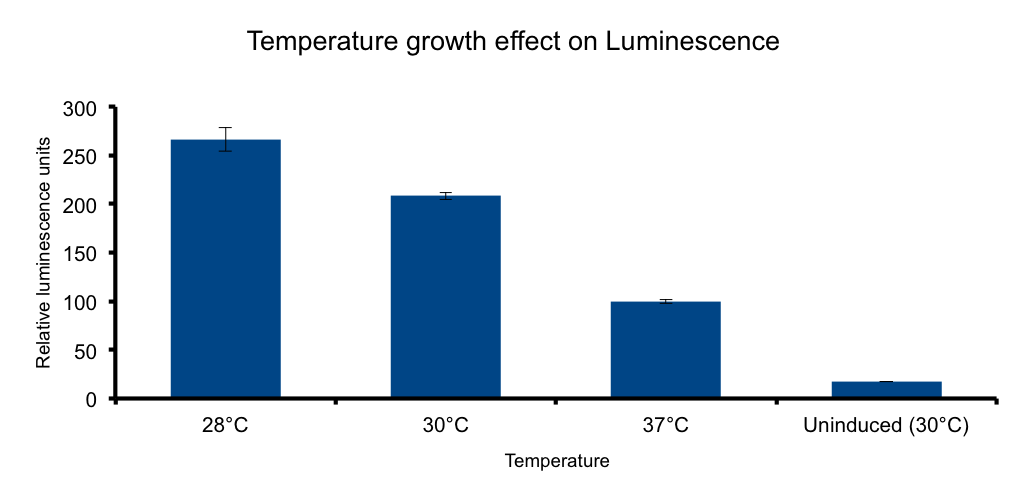
Growth temperature
Lux genes were originally cloned from Vibrio fischeri, a free living bacteria found in tropical sea water that also inhabits the poikilotherm mollusc Euprymna scolopes body in a symbiotic relationship, therefore we expect the Lux genes performance to be functional in the range of temperatures found in that context.
It has been shown that the optimal temperature conditions for bioluminescence in V. harveyi range from 20-26°C 4 and the LuxBrick page states that luminescence is diminished in temperatures above 30°. We aimed to better characterize the luminiscent response in a set of temperature conditions used in E.coli cultivation.
References
(1) Makemson, J C. Luciferase-dependent oxygen consumption by bioluminescent vibrios.
J. Bacteriol. February 1986 vol. 165 no. 2 461-466
(2) Ulitzur, S. A. Reinhertz and J. W. Hastings.Factors affecting the cellular expression of bacterial luciferase
Archives of Microbiology. Volume 129, Number 1 (1981), 67-71
(3) Rahman, Mahbuba; Rubayet, Mohammad; Hasan and Kazuyuki Shimizu.Growth phase-dependent changes in the expression of global regulatory genes and associated metabolic pathways in Escherichia coli.Biotechnology letters.
Volume 30, Number 5 (2008)
</div>
(4) Chang, Dong-Eun; Darren J. Smalley; Tyrrell Conway. Gene expression profiling of Escherichia coli growth transitions: an expanded stringent response model. Molecular Microbiology. Volume 45, Issue 2, pages 289–306, July 2002.
(5) Scheerer S, Gomez F, Lloyd D. Bioluminescence of Vibrio fischeri in continuous culture: optimal conditions for stability and intensity of photoemission. Journal of Microbiol Methods. 2006 Nov;67(2):321-9. Epub 2006 Jun 5.
 "
"