Team:UC Chile/Results/LuxBrick
From 2012.igem.org
(Difference between revisions)
Juan Alamos (Talk | contribs) (→Glucose concentration in media) |
Juan Alamos (Talk | contribs) |
||
| Line 26: | Line 26: | ||
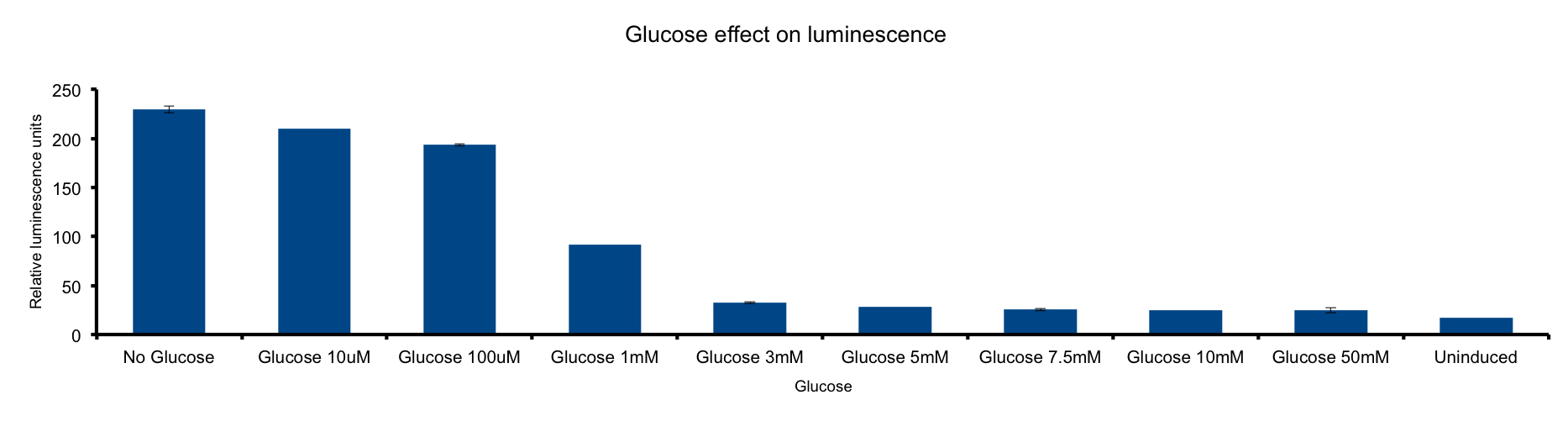
Glucose is a molecule central for the energy metabolism throughout living organisms and its the main energy source for E.coli. | Glucose is a molecule central for the energy metabolism throughout living organisms and its the main energy source for E.coli. | ||
As reduced carbon-hydrogen bonds in glucose are oxidized through glicolisis and TCA cycle, reduced equivalents are stored in NADPH and FADH2. The majority of these electrons finally react with molecular oxygen to form water at the end of the electron transport chain (ETC), reaction that is catalized by citochrome c oxydase. | As reduced carbon-hydrogen bonds in glucose are oxidized through glicolisis and TCA cycle, reduced equivalents are stored in NADPH and FADH2. The majority of these electrons finally react with molecular oxygen to form water at the end of the electron transport chain (ETC), reaction that is catalized by citochrome c oxydase. | ||
| - | This metabolic pathway is linked to the Lux light emition in two ways: the luciferase reaction consumes reducing power and in the other hand it can be considered respiration as it consumes oxygen | + | This metabolic pathway is linked to the Lux light emition in two ways: the luciferase reaction consumes reducing power and in the other hand it can be considered respiration as it consumes oxygen [[#1|1]]. |
Our results clearly show a strong inhibition of the light emition down to non-induced levels at glucose concentrations higher than 3mM . Our hypothesis is that ETC respiration competes for oxygen with luciferase respiration, then, if glucose is added to de medium, the reduction of oxygen to water would be enhanced, making oxygen less available to luciferase. | Our results clearly show a strong inhibition of the light emition down to non-induced levels at glucose concentrations higher than 3mM . Our hypothesis is that ETC respiration competes for oxygen with luciferase respiration, then, if glucose is added to de medium, the reduction of oxygen to water would be enhanced, making oxygen less available to luciferase. | ||
| - | The fact that LuxAB light emittion is augmented by cytochrome c ocydase inhibition supports this idea. | + | The fact that LuxAB light emittion is augmented by cytochrome c ocydase inhibition supports this idea [[#2|2]]. |
[[File:UC_Chile-Glucose_effect_on_luminescence_.png|962px]] | [[File:UC_Chile-Glucose_effect_on_luminescence_.png|962px]] | ||
</div> | </div> | ||
| Line 35: | Line 35: | ||
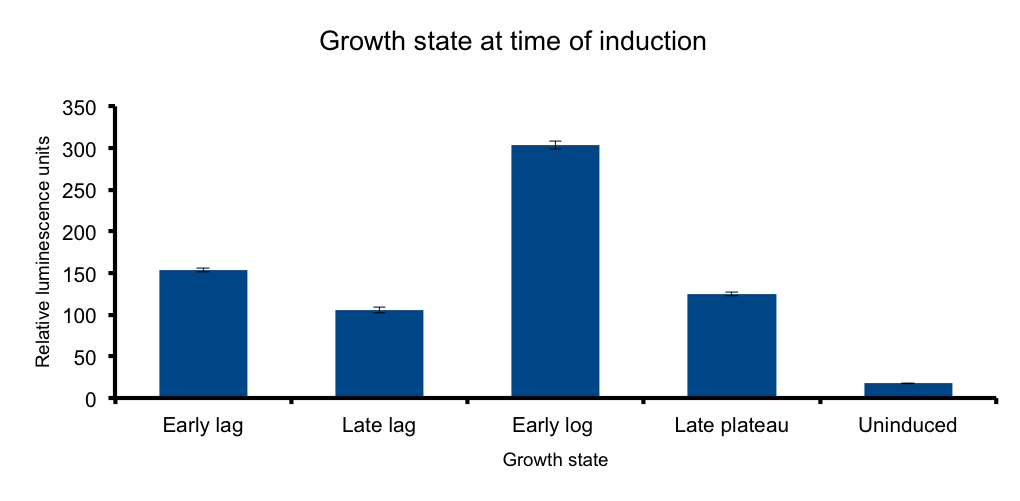
== Growth state at-time-of-induction == | == Growth state at-time-of-induction == | ||
| - | It has been know for some time that general bacterial metabolism is determined by its growth state | + | It has been know for some time that general bacterial metabolism is determined by its growth state [[#3|3]]. Growth phase determines available resources and stress derived restrictions. Bacteria can dramatically adjust their metabolism to adapt to these changing conditions. |
At the transcriptional level, different sigma subunits present at different growth states regulate gene expression throughout the whole genome(ref3) | At the transcriptional level, different sigma subunits present at different growth states regulate gene expression throughout the whole genome(ref3) | ||
We hipotezised that an heterologous metabolic pathway such as Lux light emittion wouldn´t be free from this regulation. | We hipotezised that an heterologous metabolic pathway such as Lux light emittion wouldn´t be free from this regulation. | ||
| Line 78: | Line 78: | ||
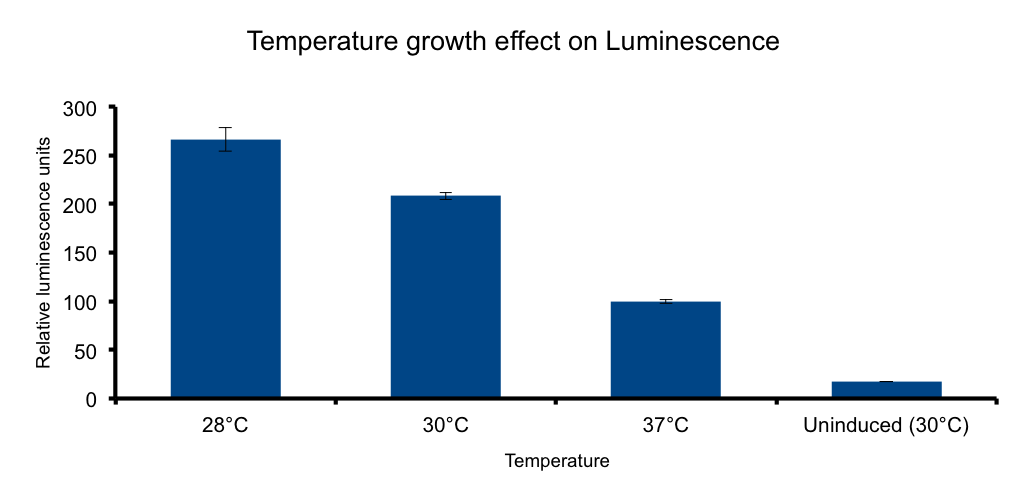
Lux genes were originally cloned from Vibrio fischeri, a free living bacteria found in tropical sea water that also inhabits the poikilotherm mollusc Euprymna scolopes body in a symbiotic relationship, therefore we expect the Lux genes performance to be functional in the range of temperatures found in that context. | Lux genes were originally cloned from Vibrio fischeri, a free living bacteria found in tropical sea water that also inhabits the poikilotherm mollusc Euprymna scolopes body in a symbiotic relationship, therefore we expect the Lux genes performance to be functional in the range of temperatures found in that context. | ||
| - | It has been shown that the optimal temperature conditions for bioluminescence in V. harveyi range from 20-26°C | + | It has been shown that the optimal temperature conditions for bioluminescence in V. harveyi range from 20-26°C [[#4|4]] and the LuxBrick page states that luminescence is diminished in temperatures above 30°. We aimed to better characterize the luminiscent response in a set of temperature conditions used in E.coli cultivation. |
[[File:UC_Chile-Temperature_growth_effect_on_Luminescence_.png|462px| right]] | [[File:UC_Chile-Temperature_growth_effect_on_Luminescence_.png|462px| right]] | ||
</div> | </div> | ||
| Line 85: | Line 85: | ||
<br> | <br> | ||
<br> | <br> | ||
| - | |||
<br> | <br> | ||
<br> | <br> | ||
<br> | <br> | ||
| + | <br><br> | ||
<br> | <br> | ||
| + | <h1>References</h1> | ||
| + | <div id="1"> | ||
| + | (1) Makemson, J C. Luciferase-dependent oxygen consumption by bioluminescent vibrios. | ||
| + | J. Bacteriol. February 1986 vol. 165 no. 2 461-466 | ||
| + | <div id="2"> | ||
| + | </div> | ||
| + | (2) Ulitzur, S. A. Reinhertz and J. W. Hastings.Factors affecting the cellular expression of bacterial luciferase | ||
| + | Archives of Microbiology. Volume 129, Number 1 (1981), 67-71 | ||
| + | </div> | ||
| + | <div id="3"> | ||
| + | </div> | ||
| + | (3) Rahman, Mahbuba; Rubayet, Mohammad; Hasan and Kazuyuki Shimizu.Growth phase-dependent changes in the expression of global regulatory genes and associated metabolic pathways in Escherichia coli.Biotechnology letters. | ||
| + | Volume 30, Number 5 (2008) | ||
| + | </div> | ||
| + | |||
| + | <br /> | ||
{{UC_Chilefooter}} | {{UC_Chilefooter}} | ||
Revision as of 22:27, 26 September 2012
 "
"