Team:Buenos Aires/Results/Bb2
From 2012.igem.org
(→Complete construct sequence and chosen assembly method) |
(→Complete construct sequence and chosen assembly method) |
||
| Line 139: | Line 139: | ||
| + | {| class="wikitable" border="1" | ||
| + | |- | ||
| + | |<!--column1-->[[File:Bsas2012-sqbb5.png|500px]] | ||
| + | |- | ||
| + | |Picture: Different Reading Frames of BBa_B0024. Frames 1 and 3 are useful as double terminators. | ||
| + | |} | ||
This construct should be able to export histidine, for sure in cyanobacterium and to be tested in E.Coli and Yeast. | This construct should be able to export histidine, for sure in cyanobacterium and to be tested in E.Coli and Yeast. | ||
Revision as of 14:33, 26 September 2012

Contents |
Construct Desing based on Biobrick Registry Parts
His Secretion in Bacteria
In the spirit of the competition we decided to design an extra construct besides our main biobricks. For creating this biobrick we decided to use solely parts that came in the iGEM Kit 2012 Spring distribution, and that could work for the same purposes that our main biobrick, which is mainly the export of aminoacids.
We designed a plausible construct that could work for the export of His using 5 parts of the registry, but we didnt find enough parts in order to design a simmilar one for the export of Trp.
Furthermore, the binding and preparation of this device is much more complex and has many more steps than what our main biobrick would require to work.
Therefore, we conclude that our main biobrick is an important contribution to the registry part, given that it allows the export of aminoacids His and Trp to be enhanced with the use of only one part, without the need of the many steps that we describe in this section and consequently reducing the risk of failure and errors.
Aim
To create a biobrick that would enhance Histidine secretion in E. coli using standard parts of the registry as a proof that one can increase the production and secretion of an aminoacid and its measurement in the culture medium.
To learn how to use and merge standard parts from the registry provided at the iGEM Kit.
To characterize the functioning of existent parts in the registry and new combinations of them, therefore contributing the improvement of the iGEM record.
To proove that our main biobricks, devices 1 and 3 for the export of His, are an important contribution to iGEM, given that they are capable of His export with far less steps involved.
To proove that our main biobricks, devices 2 and 4 for the export of Trp, are an important contribution to iGEM, given that there are was no biobrick with such function available.
Parts to use
In order to create this biobrick we would need a promoter (constitutive or inducible, a ribosome binding site(RBS),a peptide signal or secretion tag so that the aminoacid is exported out of the cell, a histidine tag (repeated several times in order to have a long peptide enriched in his) and a terminator.
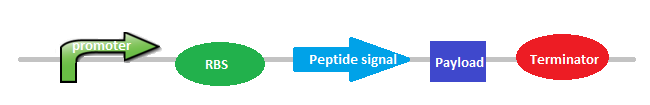
|
| Picture: General Design of Biobrick 5. |
Promoter choice
We found many usable parts to use as promoters. We found Promoters + RBS ideal for our purposes, in order to economize ligation steps.
We considered using two Biobricks:
1) Promoter + RBS: BBa_K206015 Strongest constitutive promoter in J23100 family (J23100) + mid-strength RBS from the community collection (B0030, 0.6.
It looks like a reliable sequence but it has not been tested according to the registry.
2) Inducible Promoter (IPTG) + RBS (Strong): BBa_J04500
This part has been tested and according to the registry it works well.
Sequence: caatacgcaaaccgcctctccccgcgcgttggccgattcattaatgcagctggcacgacaggtttcccgactggaaagcgggcagtgagcgcaacgcaat taatgtgagttagctcactcattaggcaccccaggctttacactttatgcttccggctcgtatgttgtgtggaattgtgagcggataacaatttcacaca tactagagaaagaggagaaa
We finally decided to use Biobrick 2: BBa_J04500, in order to make our system plausible of regulation through IPTG.
This kind of regulation could also have been implemented in our main biobricks 1 and 3, for the same purposes or making the system more flexible.
Signal Peptide Options
Unfortunately, we found very few signal peptide biobrick options, solely two and tested in Cyanobacterium.
Our two options were:
1) pilA1 signal sequence from cyanobacterium Synechocystis; secretes protein: BBa_K125300
Sequence:
tggctagtaattttaaattcaaactcctctctcaactctccaaaaaacgggcagaaggtggt
2) slr2016 signal sequence from cyanobacterium Synechocystis; secretes protein: BBa_K125310
Sequence:
tggcagcaaaacaactatggaaaattttcaatcctagaccgatgaagggtgga
These parts are only partically confirmed and optimized for working in Cyanobacterium, not E. coli or Yeast. We could use any of them in order to test them but not having any E.coli or yeast optimized signal peptide available at the registry is a critical obstacle in the project.
We would use Biobrick 2: BBa_K125310.
HisTag (Payload) Options
The only option available in iGEM Kit Spring distribution 2012 was:
Methionine + His Affinity Tag x 6: BBa_K133035 This part is only partially confirmed.
We would put this 3 times in a row:
Sequence: atgcaccaccaccaccaccacatgcaccaccaccaccaccacatgcaccaccaccaccaccac
Note: It would take 3 ligation steps to obtain a larger peptide enriched Histidine, whereas in our main biobrick 1 and 3, it already comes in the same construct.
Terminator
There are several options for terminators, but we would use a double terminator in order to be sure that it works.
Double terminador: BBa_B0024
This sequence has a double terminator in several of reading frames, which could be very useful.
Sequence:
aaataataaaaaagccggattaataatctggctttttatattctctctctagtatataaacgcagaaaggcccacccgaaggtgagccagtgtga
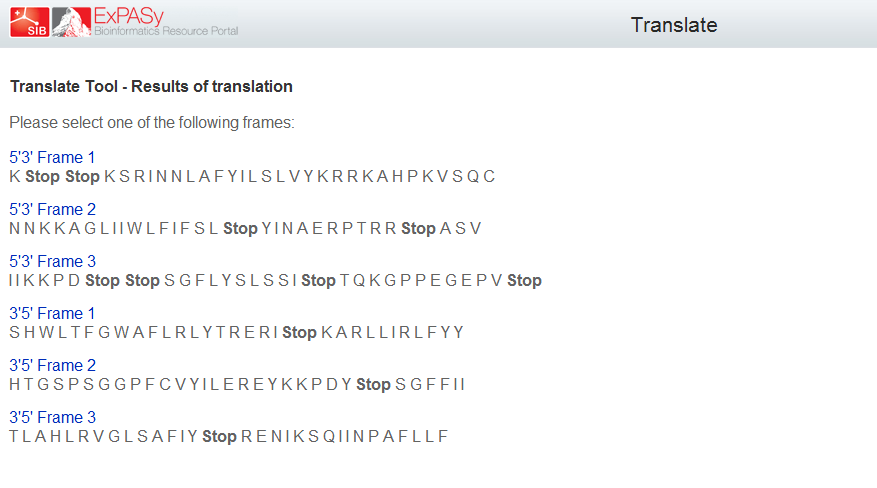
|
| Picture: Different Reading Frames of BBa_B0024. Frames 1 and 3 are useful as double terminators. |
Complete construct sequence and chosen assembly method
All the chosen parts are compatible with RFC 21 Standard, which is an in frame assembly method and would be our choice for assembling these parts.
Therefore the final sequence with prefix, suffix and scars would be:
| 500px |
| Picture: Different Reading Frames of BBa_B0024. Frames 1 and 3 are useful as double terminators. |
This construct should be able to export histidine, for sure in cyanobacterium and to be tested in E.Coli and Yeast.
 "
"