Team:Cambridge/Lab book/Week 5
From 2012.igem.org
(→Friday (27/07/12)) |
|||
| (One intermediate revision not shown) | |||
| Line 13: | Line 13: | ||
!align="center"|[[Team:Cambridge/Lab_book/Week_11|11]] | !align="center"|[[Team:Cambridge/Lab_book/Week_11|11]] | ||
!align="center"|[[Team:Cambridge/Lab_book/Week_12|12]] | !align="center"|[[Team:Cambridge/Lab_book/Week_12|12]] | ||
| + | !align="center"|[[Team:Cambridge/Lab_book/Week_13|13]] | ||
| + | !align="center"|[[Team:Cambridge/Lab_book/Week_14|14]] | ||
|} | |} | ||
| Line 143: | Line 145: | ||
| - | '''[[Team:Cambridge/Protocols/GelExtractionofDNA|Purification of successfully amplified DNA from gel]]''' | + | '''Ribosense & Ratiometrica: [[Team:Cambridge/Protocols/GelExtractionofDNA|Purification of successfully amplified DNA from gel]]''' |
---- | ---- | ||
Latest revision as of 15:22, 14 September 2012
| Week: | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|
Contents |
Monday (23/07/12)
Tuesday (24/07/12)
Wednesday (25/07/12)
Thursday (26/07/12)
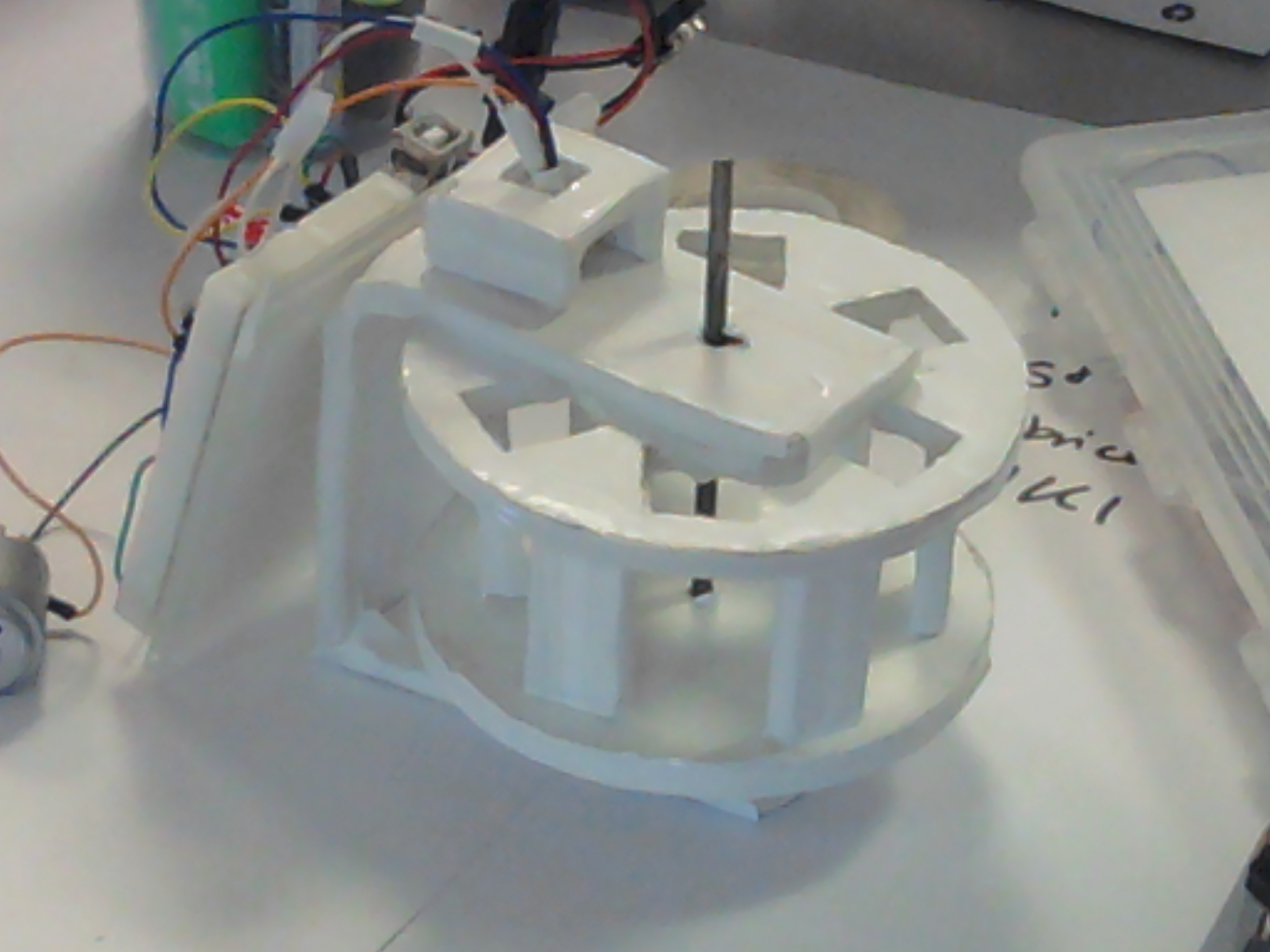
Instrumentation: Hardware implementation
Our device is close to being implemented. The filtered photoresistors are placed now in the right position, the motory circuit is also functioning, the main chassis of the device is constructed and painted. However, the mirrors which will be used to surround the cuvettes are still missing and so is the mechanical coupler designed by us and being constructed at the Instrument shop of the Engineering Department. The device is also powered by a 9V battery, without the need to be connected to the computer.
Ratiometrica: PCR of vectors, CFP, YFP, Promotor, Terminator and mOrange
- Plasmid PJS 130 used as our backbone for isolating the magnesium riboswitch and constructing the fluorescent ratiometric plasmid.
- Parts from registry amplified: E0020 (CFP), B0015 (Terminator), K143083 (Pveg) and E0030 (YFP).
- mOrange from lab also used
- Primers:
- E0020
- Forward: cacaattaaaggaggaattcaaa|ATGGTGAGCAAGGGC
- Reverse: tcgttttatttgatgcctgg|TTATTACTTGTACAGCTCGTCCA
- B0015
- Forward: acgagctgtacaagtaataa|CCAGGCATCAAATAAAACGA
- Reverse: aaaattattttgacaaaatt|TATAAACGCAGAAAGGCCC
- K143083
- Forward: tgggcctttctgcgtttata|AATTTTGTCAAAATAATTTTATTG
- Reverse: tcctcgcccttgctcaccat|ctagta|TTCACCACCTTTCTCTAGTAACA
- E0030
- Forward: tgttactagagaaaggtggtgaa|tactag|ATGGTGAGCAAGGGCG
- Reverse: gatgcctggctctagtatca|TTATTACTTGTACAGCTCGTCCA
- mOrange
- Forward: accaaaaaggaatagagt|ATGGTGAGCAAGGGCG
- Reverse: caaacttcat|ACTTCCTCCTCCTCCACTTCCTCCTCCTCC|cttgtaagctcgtccatgc
- pJS130 Mg2+
- Forward: tagaggaggtacgagtcccg|ATGAAACCAGTAACGTTATACGA
- Reverse: tacatcacaattacggaaca|CAAAATCGTCTCCCTCC
- pJS130 Fluorescent
- Forward: acgagctgtacaagtaataa|TGATACTAGAGCCAGGCATC
- Reverse: tcctcgcccttgctcaccat|TTTGAATTCCTCCTTTAATT
- PCR settings:
- 95 °C - 6mins
- 98 °C - 10secs
- 58 °C - 45secs
- 72 °C - 180secs
- Repeat above 35x
- 72 °C - 5mins
- 25 °C - 1min
PCR of riboswitch DNA from bacillus genome and Lux genes from e.coli genome
- Colony of strain pJS130 used as template for riboswitch, colony of previously transformed e.coli used as template for lux operon.
- M2+ RS
- Forward: cggagggagacgattttg|TGTTCCGTAATTGTGATGTAAG
- Reverse: tataacgttactggtttcat|CGGGACTCGTACCTCC
- Lux operon
- Forward: gctgtacaag|GGAGGAGGAGGAAGTGGAGGAGGAGGAAGT|atgaagtttggaaatatttgtttttc
- Reverse: cctcgcccttgctcaccat|ACTCTATTCCTTTTTGGTGATTC
- PCR settings - as above (run in parallel).
Friday (27/07/12)
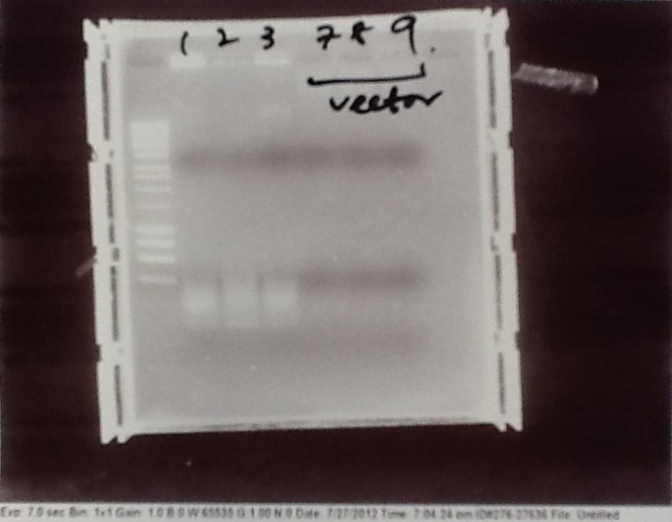
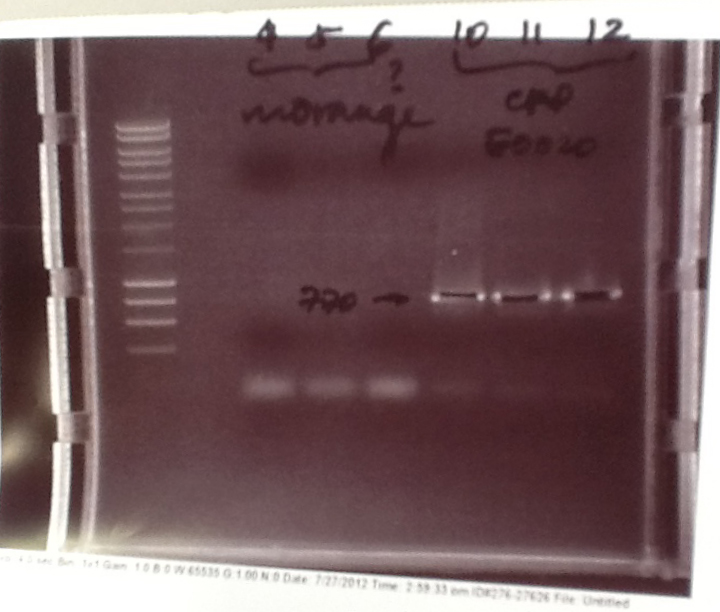
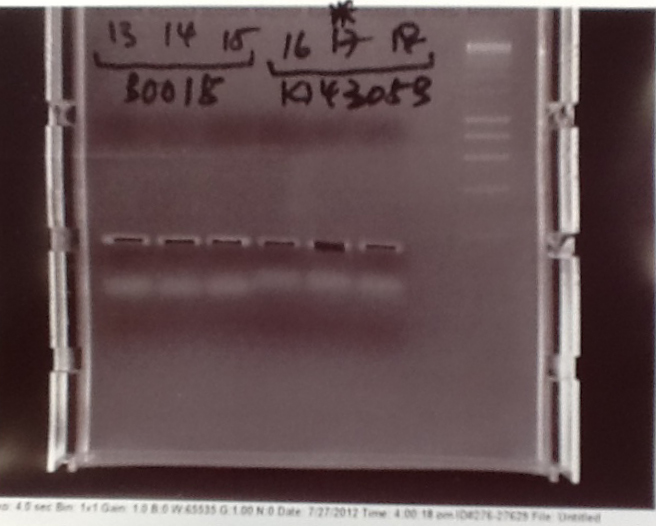
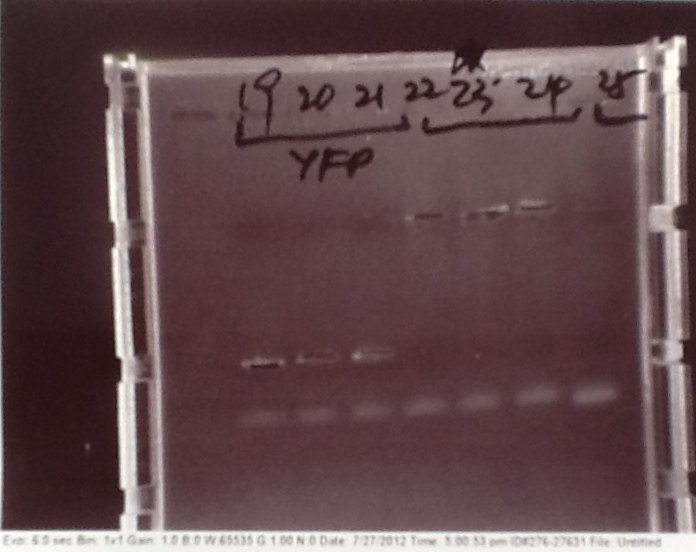
Ribosense & Ratiometrica: Gel electrophoresis of PCR products
- PCR fragments for Mg2+ riboswitch, luxA/mOrange fusion and fluorescent construct from yesterday separated on gels. 90mins at 100V.
- Mostly successful, but will need to repeat four runs: Three vectors (fluorescent, fusion (lux) and Mg2+ riboswitch (-8 codons) and mOrange gene.
Ribosense & Ratiometrica: Purification of successfully amplified DNA from gel
- Successful CFP, YFP, B0015, Pveg + SpoVG (K143053), riboswitch and vector DNA extracted from gel and purified using a minelute column. DNA frozen for later Gibson assembly.
 "
"