Team:UT-Tokyo/Project/Inhibition/Discussion
From 2012.igem.org
m |
m |
||
| (5 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
<!-- subpagename=の後の「ページ名」部分に、このページの名称を記述してください。 --> | <!-- subpagename=の後の「ページ名」部分に、このページの名称を記述してください。 --> | ||
<html> | <html> | ||
| - | </p><img id="abstimg" src="https://static.igem.org/mediawiki/2012/ | + | </p><img id="abstimg" src="https://static.igem.org/mediawiki/2012/2/27/UT_Tokyo_RFP-2.jpg" style="opacity:0.4" height="340" alt="box-background image" /><p> |
<!-- ここから</p>の前までを編集してください --> | <!-- ここから</p>の前までを編集してください --> | ||
</p></div><p></html> | </p></div><p></html> | ||
<!-- 以下テンプレ部分まで自由記述 --> | <!-- 以下テンプレ部分まで自由記述 --> | ||
| - | == | + | ==LacI - pLac== |
LacI represses the frequently used promoter, pLac. The binding of LacI to pLac is competitively inhibited and the repression of pLac is weakened by introducing 8 LacI-binding-sites on pSB1C3 (a high-copy-plasmid). | LacI represses the frequently used promoter, pLac. The binding of LacI to pLac is competitively inhibited and the repression of pLac is weakened by introducing 8 LacI-binding-sites on pSB1C3 (a high-copy-plasmid). | ||
By introducing 8 tandem repeats of LacI binding sites on pSB1C3 in addition to pLac-RBS-GFP-d.term on pSB1A2, it is expected that the binding of LacI to pLac is competitively inhibited which can be visually tracked by GFP expression. Moreover, longer LacI binding sites (more than 8 binding sites) is thought to capture more LacI and enhance GFP expression more effectively. | By introducing 8 tandem repeats of LacI binding sites on pSB1C3 in addition to pLac-RBS-GFP-d.term on pSB1A2, it is expected that the binding of LacI to pLac is competitively inhibited which can be visually tracked by GFP expression. Moreover, longer LacI binding sites (more than 8 binding sites) is thought to capture more LacI and enhance GFP expression more effectively. | ||
| - | == | + | ==ArgR - ArgR binding site== |
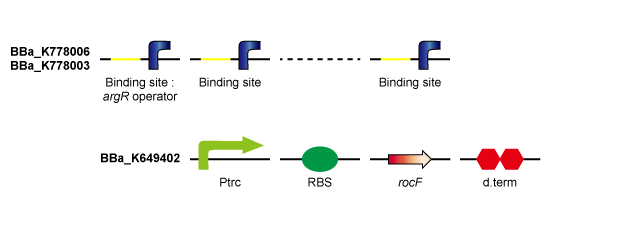
rocF is the gene coding the enzyme that converts L-arginine to L-ornithine and urea. By introducing rocF, e.coli obtains the urea cycle. ArgR is the common repressor of the bacterial arginine biosynthetic genes. By introducing ArgR binding sites, the probability that ArgR binds to the operator of the arginine biosynthetic genes falls and arginine biosynthesis is derepressed. (Tokyo Tech 2011) | rocF is the gene coding the enzyme that converts L-arginine to L-ornithine and urea. By introducing rocF, e.coli obtains the urea cycle. ArgR is the common repressor of the bacterial arginine biosynthetic genes. By introducing ArgR binding sites, the probability that ArgR binds to the operator of the arginine biosynthetic genes falls and arginine biosynthesis is derepressed. (Tokyo Tech 2011) | ||
By introducing ArgR binding sites (including 8 binding sites) on pSB1C3 (high-copy-plasmid) in addition to Ptrc-RBS-rocF on pSB6A1 (low-copy-plasmid), ArgR is sequestered by ArgR binding sites and arginine biosynthesis is derepressed, so that urea production rate is expected to rise compared to Ptrc-RBS-rocF on low-copy-plasmid / ArgR binding site on high-copy-plasmid (Tokyo Tech 2011). Furthermore, although a single ArgR binding site introduced downstream of Ptrc-RBS-rocF did not derepress arginine biosynthesis, it is thought that sufficiently long ArgR binding sites introduced downstream the Ptrc-RBS-rocF binds to many more ArgR proteins and derepresses arginine biosynthesis, consequently enhancing the urea production rate. | By introducing ArgR binding sites (including 8 binding sites) on pSB1C3 (high-copy-plasmid) in addition to Ptrc-RBS-rocF on pSB6A1 (low-copy-plasmid), ArgR is sequestered by ArgR binding sites and arginine biosynthesis is derepressed, so that urea production rate is expected to rise compared to Ptrc-RBS-rocF on low-copy-plasmid / ArgR binding site on high-copy-plasmid (Tokyo Tech 2011). Furthermore, although a single ArgR binding site introduced downstream of Ptrc-RBS-rocF did not derepress arginine biosynthesis, it is thought that sufficiently long ArgR binding sites introduced downstream the Ptrc-RBS-rocF binds to many more ArgR proteins and derepresses arginine biosynthesis, consequently enhancing the urea production rate. | ||
| Line 111: | Line 111: | ||
{{:Team:UT-Tokyo/Template/Side|twdisp=block}} | {{:Team:UT-Tokyo/Template/Side|twdisp=block}} | ||
<!-- 以下サイドメニュー編集 ;の後には、もしこのページが第2階層にあれば、そのページ内の見出しを羅列。第1階層にあれば、そのカテゴリ内の第2階層ページ名を羅列する。;の後には名称を、:の後にはその説明を記述する。:と;のセットは適当に増減してください --> | <!-- 以下サイドメニュー編集 ;の後には、もしこのページが第2階層にあれば、そのページ内の見出しを羅列。第1階層にあれば、そのカテゴリ内の第2階層ページ名を羅列する。;の後には名称を、:の後にはその説明を記述する。:と;のセットは適当に増減してください --> | ||
| - | ;[[# | + | ;[[#LacI - pLac|LacI - pLac]] |
: | : | ||
| - | ;[[# | + | ;[[#ArgR - ArgR binding site|ArgR - ArgR binding site]] |
: | : | ||
;[[#Modeling|Modeling]] | ;[[#Modeling|Modeling]] | ||
| - | + | *[[#For LacI|For LacI]] | |
| + | *[[#With Larger/Smaller k Than That of LacI|With Larger/Smaller k Than That of LacI]] | ||
| + | *[[#Reference|Reference]] | ||
| + | |||
<!-- ここまでサイドメニュー編集を --> | <!-- ここまでサイドメニュー編集を --> | ||
<html></p> | <html></p> | ||
Latest revision as of 03:09, 27 September 2012
Inhibition without Knockout:
Results & Discussion

LacI - pLac
LacI represses the frequently used promoter, pLac. The binding of LacI to pLac is competitively inhibited and the repression of pLac is weakened by introducing 8 LacI-binding-sites on pSB1C3 (a high-copy-plasmid). By introducing 8 tandem repeats of LacI binding sites on pSB1C3 in addition to pLac-RBS-GFP-d.term on pSB1A2, it is expected that the binding of LacI to pLac is competitively inhibited which can be visually tracked by GFP expression. Moreover, longer LacI binding sites (more than 8 binding sites) is thought to capture more LacI and enhance GFP expression more effectively.
ArgR - ArgR binding site
rocF is the gene coding the enzyme that converts L-arginine to L-ornithine and urea. By introducing rocF, e.coli obtains the urea cycle. ArgR is the common repressor of the bacterial arginine biosynthetic genes. By introducing ArgR binding sites, the probability that ArgR binds to the operator of the arginine biosynthetic genes falls and arginine biosynthesis is derepressed. (Tokyo Tech 2011) By introducing ArgR binding sites (including 8 binding sites) on pSB1C3 (high-copy-plasmid) in addition to Ptrc-RBS-rocF on pSB6A1 (low-copy-plasmid), ArgR is sequestered by ArgR binding sites and arginine biosynthesis is derepressed, so that urea production rate is expected to rise compared to Ptrc-RBS-rocF on low-copy-plasmid / ArgR binding site on high-copy-plasmid (Tokyo Tech 2011). Furthermore, although a single ArgR binding site introduced downstream of Ptrc-RBS-rocF did not derepress arginine biosynthesis, it is thought that sufficiently long ArgR binding sites introduced downstream the Ptrc-RBS-rocF binds to many more ArgR proteins and derepresses arginine biosynthesis, consequently enhancing the urea production rate.
Modeling
To assess the effectiveness of our method, we made a model and to calculate estimated differences in transcription factor availability with and without adding tandem repeats of binding sites.
We start with up the equilibrium reaction:
By introducing binding sites into high-copy plasmids, the following reactions are added:
Let
- [I]:=concentration of the transcription factor alone
- [G]:=concentration of the genomic DNA alone
- [GI]:=concentration of the transcription factor - genome DNA complex
- [T]:=concentration of the binding site on plasmid alone
- [TI]:concentration of the transcriptional factor - binding site complex
Also, we assumed that the equilibrium constants, k, are equal.
![]()
This leads to
- [GI] = k [I][G]
- [TI] = k [I][T]
- [I]+[GI]+[TI]=const=:X (Total concentration of the transcription factor)
- [G]+[GI]=const=:Y (Total concentration of the genomic DNA)
- [T]+[TI]=const=:Z (Total concentration of the binding site on plasmid)
What we want to know is how ![]() changes from a situation where [G]:[T] is 1:0 (wild type) to 1:1000 (introducing 10 tandem binding sites into a 100 copy plasmid), because
changes from a situation where [G]:[T] is 1:0 (wild type) to 1:1000 (introducing 10 tandem binding sites into a 100 copy plasmid), because ![]() represents the proportion of Genomic DNA bound by the transcription factor. Let u:=
represents the proportion of Genomic DNA bound by the transcription factor. Let u:= ![]() .
.
we set the variables y:=Y/X, z:=Z/X, t:=1/kX.
For LacI
Now, we solve this equation for the LacI transcription factor because its k is known.
the equilibrium constant ![]() for the equilibrium [1]
for the equilibrium [1]
The concentration of genomic DNA is ![]() [2]
[2]
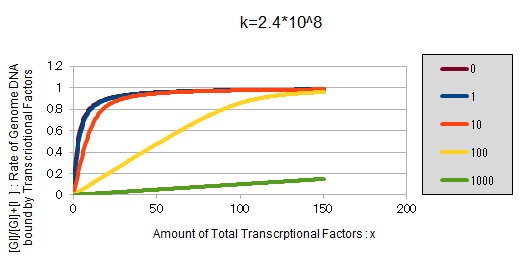
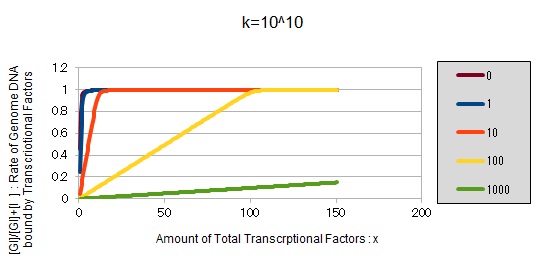
From the above, we caluculated u against X (Total concentration of the transcription factor) for several values of Z/Y(the number of binding sites introduced) = 0, 1, 10, 100, 1000 and obtained the graph following
This graph shows the obvious effectiveness of introducing LacI binding sites into a high-copy plasmid (when the copy number ~ 10^2). Also, it shows that If X is high the effect of introducing tandem repeat is greater(by comparing Z/Y = 100 and 1000).
With Larger/Smaller k Than That of LacI
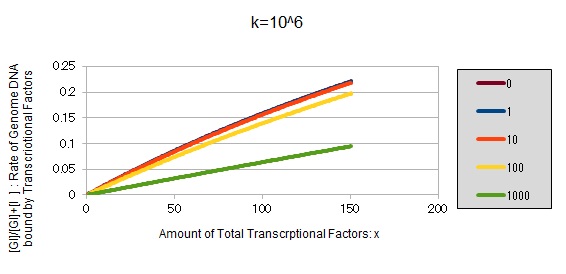
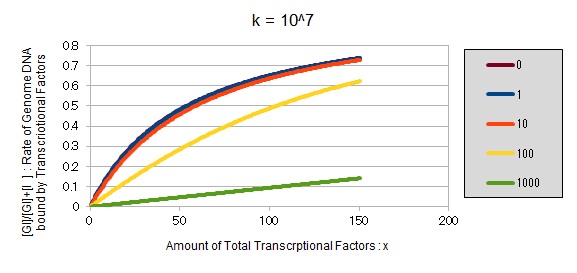
For reference, we write some graphs with larger/smaller k, to apply to other transcription factors.
If k==10^6:
If k==10^7:
If k==2.4 * 10^8 (LacI)
If k==10^10:
These graphs show that as k is increased, the effect of introducing binding sites becomes bigger.
Reference
- [1] LactoseRepressor-OperatorDNA Interactions: KineticAnalysis by aSurfacePlasmonResonanceBiosensor K. Bondeson, A. Frostellkarlsson, L. Fagerstam, G. Magnusson Univ Uppsala, Ctr Biomed, Dept Med Virol, S 75123 Uppsala, Sweden and Pharmacia Biosensor AB, S 75182 Uppsala, Sweden
- [2] CCDB E.coli stastics http://ccdb.wishartlab.com/CCDB/cgi-bin/STAT_NEW.cgi
 "
"