Team:ETH Zurich/Design
From 2012.igem.org
| (31 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:ETH_Zurich/Templates/TestHeader}} | {{:Team:ETH_Zurich/Templates/TestHeader}} | ||
| + | |||
| + | === Expression of the proteins PabA & PabB/C === | ||
| + | |||
| + | TOP10 colonies were transformed with <partinfo>BBa_K137055</partinfo> , <partinfo>BBa_S04039</partinfo> and <partinfo>BBa_K909014</partinfo>. The transformed cells as well as the TOP10 WT were cultivated ON in falcon tubes containing 4 mL of LB. After normalization to an OD of 0.15, the cells were lysed and mixed with 6X Laemmli buffer. | ||
| + | |||
| + | PabA has a size of 21,656 kDa (193 aa), PabB/C of 71,743 kDA (628 aa). | ||
| + | |||
| + | |||
| + | SDS PAGE | ||
| + | |||
== Design of the single parts== | == Design of the single parts== | ||
| - | |||
| - | Three different fusion strategies were carried out to verify the best UVR8 dTetR fusion [http://partsregistry.org/Part:BBa_K909008]. Tet DBD stands for TetR DNA binding domain. | + | === YcgZ promoter with multiple BluR (YcgE) operator sites === |
| + | |||
| + | One of our approaches was to make use of the Blue light sensitive protein BluF (YcgF) which is activation upon blue light radiation, then dimerizes and inactivates BluR (YcgE), a transcriptional repressor which binds to the YcgZ promoter. We cloned the ''lacZ'' downstream to the YcgZ promoter and tested the beta-galactosidase activity making use of the Miller Assay. As we could not see any difference between the different K.O. strains we suggested that the endogenous amounts of activator and repressor was not enough to lead to a clear difference. | ||
| + | |||
| + | Leakiness. Tschowri DNA footprint assay.. Identified operator sites. picture.... link to partsregistry. bliblanrfhiuqifufihuwe | ||
| + | |||
| + | |||
| + | === UVR8 - dTetR fusion: a UV sensing protein === | ||
| + | |||
| + | Three different fusion strategies were carried out to verify the best UVR8 dTetR fusion [[http://partsregistry.org/Part:BBa_K909008 link to the partsregistry]]. Tet DBD stands for TetR DNA binding domain. | ||
1. Full length UVR8 without a linker | 1. Full length UVR8 without a linker | ||
| Line 21: | Line 39: | ||
[[File:Uvr8uv.jpg|frameless|400px|center]] | [[File:Uvr8uv.jpg|frameless|400px|center]] | ||
| - | You can find the split TetR DNA binding domain containing a BamHI restriction site which allows it to be fused to any protein of interest containing the corresponding restriction site. | + | You can find the split [http://partsregistry.org/Part:BBa_K909007 TetR DNA binding domain containing a BamHI restriction site] which allows it to be fused to any protein of interest containing the corresponding restriction site. This allows you to test if a protein of interest is able to dimerize. |
| Line 29: | Line 47: | ||
=== p-ABA generator === | === p-ABA generator === | ||
| - | We constructed one operon consisting of the pabA and pabB/C gene from Lactococcus | + | We constructed one [http://partsregistry.org/Part:BBa_K909015 pab operon] consisting of the pabA and pabB/C gene from ''Lactococcus lactis'' to overexpress p-ABA. |
| - | Therefore we used the biobrick parts | + | Therefore we used the biobrick parts [http://partsregistry.org/Part:BBa_K137055 BBa_K137055] and [http://partsregistry.org/Part:BBa_S04039 BBa_S04039] as a template to construct the wished composites. |
| - | In a second step the pab operon | + | |
| + | [http://partsregistry.org/Part:BBa_K909016 Biobrick K909016] will be used to show that p-ABA is produced, while the operon can be cloned downstream to any promoter. | ||
| + | |||
| + | |||
| + | UVR8 - system: | ||
| + | In a second step the pab operon will be cloned downstream of the pTet promoter. Derepression of UVR8 by UV irradiation leads then to p-ABA expression. | ||
| + | |||
| + | Decoder: | ||
| + | |||
| + | In a second step the pab operon will be cloned downstream of the [http://partsregistry.org/Part:BBa_K909011 PL promoter with operator sites TetO<sub>1</sub> and LacO<sub>1</sub>]. In case blue and red light is present, p-ABA will be produced as a blue and red light response. | ||
=== Dual input promoters === | === Dual input promoters === | ||
| - | In our case we want the | + | In our case we want the [[Team:ETH_Zurich/Modeling/LovTAP-Cph1| decoder]] to have AND logics. Therefore we designed 3 different dual input promoters, all of them derived from the λ-phage promoters P<sub>R</sub> and P<sub>L</sub>: |
| + | |||
| + | 1. [http://partsregistry.org/Part:BBa_K909012 Modified P<sub>R</sub> promoter with operator sites O<sub>R1</sub>,O<sub>R2</sub> and LacO<sub>1</sub>] | ||
| + | |||
| + | 2. [http://partsregistry.org/Part:BBa_K909013 Modified P<sub>R</sub> promoter with operator sites O<sub>R1</sub>,O<sub>R2</sub> and TetO<sub>1</sub>] | ||
| + | |||
| + | 3. [http://partsregistry.org/Part:BBa_K909011 Modified P<sub>L</sub> promoter with operator sites TetO<sub>1</sub> and LacO<sub>1</sub>] | ||
| - | |||
| - | |||
| - | + | Truth table | |
Latest revision as of 00:19, 27 September 2012
Contents |
Expression of the proteins PabA & PabB/C
TOP10 colonies were transformed with <partinfo>BBa_K137055</partinfo> , <partinfo>BBa_S04039</partinfo> and <partinfo>BBa_K909014</partinfo>. The transformed cells as well as the TOP10 WT were cultivated ON in falcon tubes containing 4 mL of LB. After normalization to an OD of 0.15, the cells were lysed and mixed with 6X Laemmli buffer.
PabA has a size of 21,656 kDa (193 aa), PabB/C of 71,743 kDA (628 aa).
SDS PAGE
Design of the single parts
YcgZ promoter with multiple BluR (YcgE) operator sites
One of our approaches was to make use of the Blue light sensitive protein BluF (YcgF) which is activation upon blue light radiation, then dimerizes and inactivates BluR (YcgE), a transcriptional repressor which binds to the YcgZ promoter. We cloned the lacZ downstream to the YcgZ promoter and tested the beta-galactosidase activity making use of the Miller Assay. As we could not see any difference between the different K.O. strains we suggested that the endogenous amounts of activator and repressor was not enough to lead to a clear difference.
Leakiness. Tschowri DNA footprint assay.. Identified operator sites. picture.... link to partsregistry. bliblanrfhiuqifufihuwe
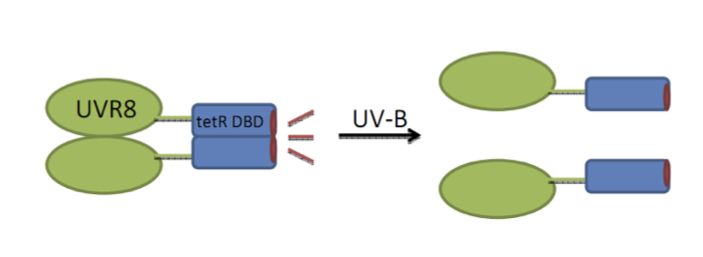
UVR8 - dTetR fusion: a UV sensing protein
Three different fusion strategies were carried out to verify the best UVR8 dTetR fusion http://partsregistry.org/Part:BBa_K909008 link to the partsregistry. Tet DBD stands for TetR DNA binding domain.
1. Full length UVR8 without a linker
2. Full length UVR8 + [GGS]x2 linker (+6 aa)
3. Truncated UVR8 (-13 aa which are not seen in the 3d structure)
You can find the split [http://partsregistry.org/Part:BBa_K909007 TetR DNA binding domain containing a BamHI restriction site] which allows it to be fused to any protein of interest containing the corresponding restriction site. This allows you to test if a protein of interest is able to dimerize.
p-ABA generator
We constructed one [http://partsregistry.org/Part:BBa_K909015 pab operon] consisting of the pabA and pabB/C gene from Lactococcus lactis to overexpress p-ABA. Therefore we used the biobrick parts [http://partsregistry.org/Part:BBa_K137055 BBa_K137055] and [http://partsregistry.org/Part:BBa_S04039 BBa_S04039] as a template to construct the wished composites.
[http://partsregistry.org/Part:BBa_K909016 Biobrick K909016] will be used to show that p-ABA is produced, while the operon can be cloned downstream to any promoter.
UVR8 - system:
In a second step the pab operon will be cloned downstream of the pTet promoter. Derepression of UVR8 by UV irradiation leads then to p-ABA expression.
Decoder:
In a second step the pab operon will be cloned downstream of the [http://partsregistry.org/Part:BBa_K909011 PL promoter with operator sites TetO1 and LacO1]. In case blue and red light is present, p-ABA will be produced as a blue and red light response.
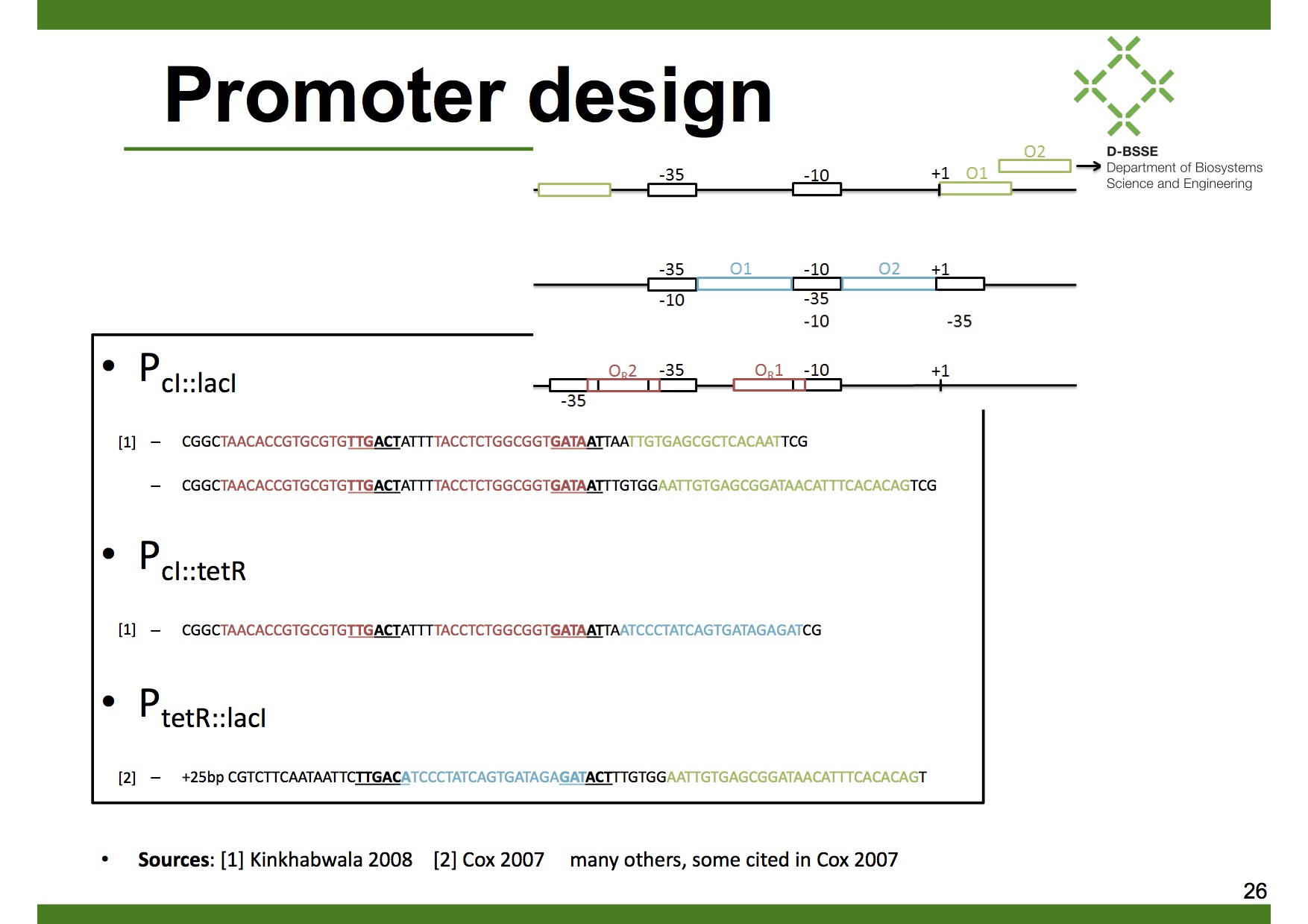
Dual input promoters
In our case we want the decoder to have AND logics. Therefore we designed 3 different dual input promoters, all of them derived from the λ-phage promoters PR and PL:
1. [http://partsregistry.org/Part:BBa_K909012 Modified PR promoter with operator sites OR1,OR2 and LacO1]
2. [http://partsregistry.org/Part:BBa_K909013 Modified PR promoter with operator sites OR1,OR2 and TetO1]
3. [http://partsregistry.org/Part:BBa_K909011 Modified PL promoter with operator sites TetO1 and LacO1]
Truth table
UVR8
UVR8
References
- Brown, B. a, Headland, L. R., & Jenkins, G. I. (2009). UV-B action spectrum for UVR8-mediated HY5 transcript accumulation in Arabidopsis. Photochemistry and photobiology, 85(5), 1147–55.
- Christie, J. M., Salomon, M., Nozue, K., Wada, M., & Briggs, W. R. (1999): LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proceedings of the National Academy of Sciences of the United States of America, 96(15), 8779–83.
- Christie, J. M., Arvai, A. S., Baxter, K. J., Heilmann, M., Pratt, A. J., O’Hara, A., Kelly, S. M., et al. (2012). Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science (New York, N.Y.), 335(6075), 1492–6.
- Cloix, C., & Jenkins, G. I. (2008). Interaction of the Arabidopsis UV-B-specific signaling component UVR8 with chromatin. Molecular plant, 1(1), 118–28.
- Cox, R. S., Surette, M. G., & Elowitz, M. B. (2007). Programming gene expression with combinatorial promoters. Molecular systems biology, 3(145), 145. doi:10.1038/msb4100187
- Drepper, T., Eggert, T., Circolone, F., Heck, A., Krauss, U., Guterl, J.-K., Wendorff, M., et al. (2007). Reporter proteins for in vivo fluorescence without oxygen. Nature biotechnology, 25(4), 443–5
- Drepper, T., Krauss, U., & Berstenhorst, S. M. zu. (2011). Lights on and action! Controlling microbial gene expression by light. Applied microbiology, 23–40.
- EuropeanCommission (2006). SCIENTIFIC COMMITTEE ON CONSUMER PRODUCTS SCCP Opinion on Biological effects of ultraviolet radiation relevant to health with particular reference to sunbeds for cosmetic purposes.
- Elvidge, C. D., Keith, D. M., Tuttle, B. T., & Baugh, K. E. (2010). Spectral identification of lighting type and character. Sensors (Basel, Switzerland), 10(4), 3961–88.
- GarciaOjalvo, J., Elowitz, M. B., & Strogatz, S. H. (2004). Modeling a synthetic multicellular clock: repressilators coupled by quorum sensing. Proceedings of the National Academy of Sciences of the United States of America, 101(30), 10955–60.
- Gao Q, Garcia-Pichel F. (2011). Microbial ultraviolet sunscreens. Nat Rev Microbiol. 9(11):791-802.
- Goosen N, Moolenaar GF. (2008) Repair of UV damage in bacteria. DNA Repair (Amst).7(3):353-79.
- Heijde, M., & Ulm, R. (2012). UV-B photoreceptor-mediated signalling in plants. Trends in plant science, 17(4), 230–7.
- Hirose, Y., Narikawa, R., Katayama, M., & Ikeuchi, M. (2010). Cyanobacteriochrome CcaS regulates phycoerythrin accumulation in Nostoc punctiforme, a group II chromatic adapter. Proceedings of the National Academy of Sciences of the United States of America, 107(19), 8854–9.
- Hirose, Y., Shimada, T., Narikawa, R., Katayama, M., & Ikeuchi, M. (2008). Cyanobacteriochrome CcaS is the green light receptor that induces the expression of phycobilisome linker protein. Proceedings of the National Academy of Sciences of the United States of America, 105(28), 9528–33.
- Kast, Asif-Ullah & Hilvert (1996) Tetrahedron Lett. 37, 2691 - 2694., Kast, Asif-Ullah, Jiang & Hilvert (1996) Proc. Natl. Acad. Sci. USA 93, 5043 - 5048
- Kiefer, J., Ebel, N., Schlücker, E., & Leipertz, A. (2010). Characterization of Escherichia coli suspensions using UV/Vis/NIR absorption spectroscopy. Analytical Methods, 9660. doi:10.1039/b9ay00185a
- Kinkhabwala, A., & Guet, C. C. (2008). Uncovering cis regulatory codes using synthetic promoter shuffling. PloS one, 3(4), e2030.
- Krebs in Deutschland 2005/2006. Häufigkeiten und Trends. 7. Auflage, 2010, Robert Koch-Institut (Hrsg) und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e. V. (Hrsg). Berlin.
- Lamparter, T., Michael, N., Mittmann, F., & Esteban, B. (2002). Phytochrome from Agrobacterium tumefaciens has unusual spectral properties and reveals an N-terminal chromophore attachment site. Proceedings of the National Academy of Sciences of the United States of America, 99(18), 11628–33.
- Levskaya, A. et al (2005). Engineering Escherichia coli to see light. Nature, 438(7067), 442.
- Mancinelli, A. (1986). Comparison of spectral properties of phytochromes from different preparations. Plant physiology, 82(4), 956–61.
- Nakasone, Y., Ono, T., Ishii, A., Masuda, S., & Terazima, M. (2007). Transient dimerization and conformational change of a BLUF protein: YcgF. Journal of the American Chemical Society, 129(22), 7028–35.
- Orth, P., & Schnappinger, D. (2000). Structural basis of gene regulation by the tetracycline inducible Tet repressor-operator system. Nature structural biology, 215–219.
- Parkin, D.M., et al., Global cancer statistics, 2002. CA: a cancer journal for clinicians, 2005. 55(2): p. 74-108.
- Rajagopal, S., Key, J. M., Purcell, E. B., Boerema, D. J., & Moffat, K. (2004). Purification and initial characterization of a putative blue light-regulated phosphodiesterase from Escherichia coli. Photochemistry and photobiology, 80(3), 542–7.
- Rizzini, L., Favory, J.-J., Cloix, C., Faggionato, D., O’Hara, A., Kaiserli, E., Baumeister, R., et al. (2011). Perception of UV-B by the Arabidopsis UVR8 protein. Science (New York, N.Y.), 332(6025), 103–6.
- Roux, B., & Walsh, C. T. (1992). p-aminobenzoate synthesis in Escherichia coli: kinetic and mechanistic characterization of the amidotransferase PabA. Biochemistry, 31(30), 6904–10.
- Strickland, D. (2008). Light-activated DNA binding in a designed allosteric protein. Proceedings of the National Academy of Sciences of the United States of America, 105(31), 10709–10714.
- Sinha RP, Häder DP. UV-induced DNA damage and repair: a review. Photochem Photobiol Sci. (2002). 1(4):225-36
- Sambandan DR, Ratner D. (2011). Sunscreens: an overview and update. J Am Acad Dermatol. 2011 Apr;64(4):748-58.
- Tabor, J. J., Levskaya, A., & Voigt, C. A. (2011). Multichromatic Control of Gene Expression in Escherichia coli. Journal of Molecular Biology, 405(2), 315–324.
- Thibodeaux, G., & Cowmeadow, R. (2009). A tetracycline repressor-based mammalian two-hybrid system to detect protein–protein interactions in vivo. Analytical biochemistry, 386(1), 129–131.
- Tschowri, N., & Busse, S. (2009). The BLUF-EAL protein YcgF acts as a direct anti-repressor in a blue-light response of Escherichia coli. Genes & development, 522–534.
- Tschowri, N., Lindenberg, S., & Hengge, R. (2012). Molecular function and potential evolution of the biofilm-modulating blue light-signalling pathway of Escherichia coli. Molecular microbiology.
- Tyagi, A. (2009). Photodynamics of a flavin based blue-light regulated phosphodiesterase protein and its photoreceptor BLUF domain.
- Vainio, H. & Bianchini, F. (2001). IARC Handbooks of Cancer Prevention: Volume 5: Sunscreens. Oxford University Press, USA
- Quinlivan, Eoin P & Roje, Sanja & Basset, Gilles & Shachar-Hill, Yair & Gregory, Jesse F & Hanson, Andrew D. (2003). The folate precursor p-aminobenzoate is reversibly converted to its glucose ester in the plant cytosol. The Journal of biological chemistry, 278.
- van Thor, J. J., Borucki, B., Crielaard, W., Otto, H., Lamparter, T., Hughes, J., Hellingwerf, K. J., et al. (2001). Light-induced proton release and proton uptake reactions in the cyanobacterial phytochrome Cph1. Biochemistry, 40(38), 11460–71.
- Wegkamp A, van Oorschot W, de Vos WM, Smid EJ. (2007 )Characterization of the role of para-aminobenzoic acid biosynthesis in folate production by Lactococcus lactis. Appl Environ Microbiol. Apr;73(8):2673-81.
 "
"