Team:ETH Zurich/Modeling/UVR8
From 2012.igem.org
Contents |
UVR8-TetRDBD
UVR8 (UV Resistance Locus 8) acts as a UVB photoreceptor in plants. It regulates the transcription factor COP and indirectly affects the expression of a myriad of genes. However, we employ it differently, as described here. In the following, we analyse the theoretical requirements for our system that employs UVR8's dimerisation ability to work. It involves some manual parameter optimisation in order to determine the optimal concentration regimes for the receptors and enzymes in the cell.
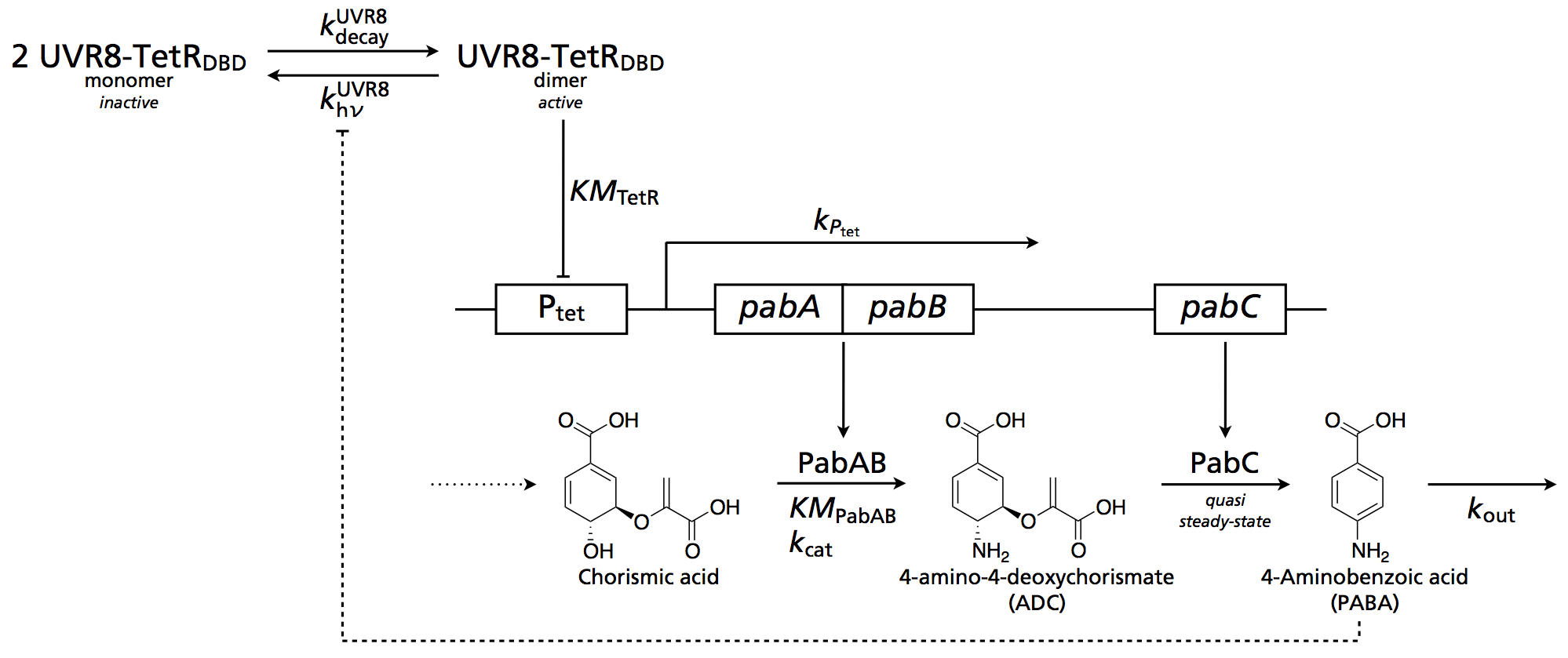
Circuit
The following scheme illustrates the system:
Modelling assumptions
In order to analyse a number of properties of the system, the model needs to be tractable. For this, a number of assumptions and approximations have been made:
- UVR8-TetRDBD is conserved. This translates to a model not explicitly accounting for UVR8-TetRDBD expression/degradation, as this will be the case for most systems in steady state anyway.
- The photoconversion process upon UV exposure of UVR8-TetRDBD is modelled as a 2-state process. While fundamentally it is highly likely that the conversion process involves a number of (meta-stable) intermediates, there is usually one rate-determining step. All other steps are then assumed to be in quasi-stationary state, thus simplifying to a 2-state system.
- Those parts of the metabolic pathway that have been cloned into our E.coli have been incorporated into our model. For this we assume the intracellular [Chorismate]cell concentrations to be constant.
- Furthermore, PABA overexpression can be achieved without upregulating pabC (aminodeoxychorismate lyase). Hence, this step is not rate-determining in PABA formation. Thus pabC has kinetics on a smaller timescale than PabAB such that 4-amino-4-deoxychorismate (ADC) concentrations are never high - justifying another quasi-stationary-assumption.
- This leads to a production term of PABA depending on the produced PabAB concentration and the assumed intracellular [Chorismate]cell concentrations and a first-order outflux term with kout.
- Lastly, PabAB is constitutively degraded with kdeg.
- We are interested in the effect of PABA on the dissociation of UVR8. As PABA can be regarded as a competitor for UVB in the cell, we model this negative feedback loop with a hill-like term in the dissociation step.
ODEs
With the aforementioned assumptions, the coupled systems boil down to:
The parameter khv and kdecay have been determined from gel assays with approximative half-lives. The promoter strength has been optimised such that we get the required enzyme concentrations. Degradation rates have been assumed from a mean half-life of 5 hours. The enzyme kinetical parameters can be found in the literature [Roux1992]. Lastly, the outflux term kout has been selected such that PABA steady-state values are comparatively large - this would involve certain metabolic optimisations in-vivo. n in the hill kinetic term with [PABA] accounts for a more or less sigmoidal behaviour of PABA inhibition.
Find the parameters we used on our parameter page.
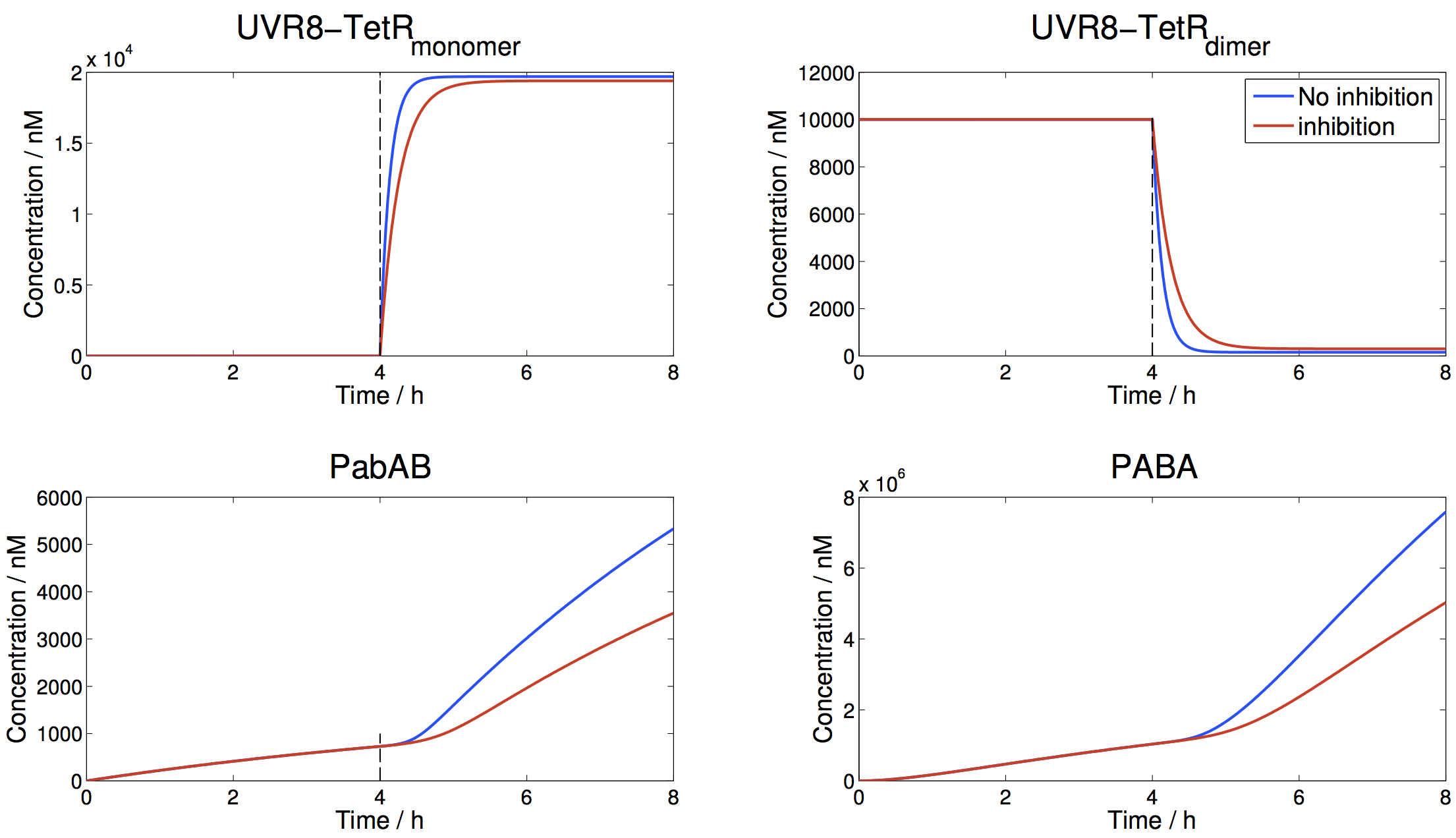
Results
Simulations have first been run for 4 hours in the dark ("inside condition"), with another 4 hours under sunny conditions. Notice the time span between first sun exposure and half-maximum of PABA production, the second sigmoidal step: This time is around 1 hour, a satisfactory time scale for the production of protective PABA for the average european caucasian after sun exposure.
Considerations
Using half-lives from in-vitro gels gives a rough figure of the timescale on which UVR8-TetRDBD dissociates. Calculating reaction parameters for UVR8 from first principles proved to be harder than expected: there are a number of consecutive chemical reactions involved in leading to a dissociated monomer, all of which we believe tend to occur on the same timescale. This clearly conflicts with an assumed 2-state model. As such, this would require an extensive photoinduction model, which is not possible at the moment, due to the exact number of reactions required not entirely known.
Another future improvement to be included will focus on improving the negative feedback part of the model. Currently this negative feedback from PABA is modelled with a Hill-like inhibition - in future this should be from first principles which will require more knowledge about how PABA UVB-absorption interferes with the receptor (UVR8) UV absorption. This might require a diffusion model of PABA when considering a layer model.
References
- Brown, B. a, Headland, L. R., & Jenkins, G. I. (2009). UV-B action spectrum for UVR8-mediated HY5 transcript accumulation in Arabidopsis. Photochemistry and photobiology, 85(5), 1147–55.
- Christie, J. M., Salomon, M., Nozue, K., Wada, M., & Briggs, W. R. (1999): LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proceedings of the National Academy of Sciences of the United States of America, 96(15), 8779–83.
- Christie, J. M., Arvai, A. S., Baxter, K. J., Heilmann, M., Pratt, A. J., O’Hara, A., Kelly, S. M., et al. (2012). Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science (New York, N.Y.), 335(6075), 1492–6.
- Cloix, C., & Jenkins, G. I. (2008). Interaction of the Arabidopsis UV-B-specific signaling component UVR8 with chromatin. Molecular plant, 1(1), 118–28.
- Cox, R. S., Surette, M. G., & Elowitz, M. B. (2007). Programming gene expression with combinatorial promoters. Molecular systems biology, 3(145), 145. doi:10.1038/msb4100187
- Drepper, T., Eggert, T., Circolone, F., Heck, A., Krauss, U., Guterl, J.-K., Wendorff, M., et al. (2007). Reporter proteins for in vivo fluorescence without oxygen. Nature biotechnology, 25(4), 443–5
- Drepper, T., Krauss, U., & Berstenhorst, S. M. zu. (2011). Lights on and action! Controlling microbial gene expression by light. Applied microbiology, 23–40.
- EuropeanCommission (2006). SCIENTIFIC COMMITTEE ON CONSUMER PRODUCTS SCCP Opinion on Biological effects of ultraviolet radiation relevant to health with particular reference to sunbeds for cosmetic purposes.
- Elvidge, C. D., Keith, D. M., Tuttle, B. T., & Baugh, K. E. (2010). Spectral identification of lighting type and character. Sensors (Basel, Switzerland), 10(4), 3961–88.
- GarciaOjalvo, J., Elowitz, M. B., & Strogatz, S. H. (2004). Modeling a synthetic multicellular clock: repressilators coupled by quorum sensing. Proceedings of the National Academy of Sciences of the United States of America, 101(30), 10955–60.
- Gao Q, Garcia-Pichel F. (2011). Microbial ultraviolet sunscreens. Nat Rev Microbiol. 9(11):791-802.
- Goosen N, Moolenaar GF. (2008) Repair of UV damage in bacteria. DNA Repair (Amst).7(3):353-79.
- Heijde, M., & Ulm, R. (2012). UV-B photoreceptor-mediated signalling in plants. Trends in plant science, 17(4), 230–7.
- Hirose, Y., Narikawa, R., Katayama, M., & Ikeuchi, M. (2010). Cyanobacteriochrome CcaS regulates phycoerythrin accumulation in Nostoc punctiforme, a group II chromatic adapter. Proceedings of the National Academy of Sciences of the United States of America, 107(19), 8854–9.
- Hirose, Y., Shimada, T., Narikawa, R., Katayama, M., & Ikeuchi, M. (2008). Cyanobacteriochrome CcaS is the green light receptor that induces the expression of phycobilisome linker protein. Proceedings of the National Academy of Sciences of the United States of America, 105(28), 9528–33.
- Kast, Asif-Ullah & Hilvert (1996) Tetrahedron Lett. 37, 2691 - 2694., Kast, Asif-Ullah, Jiang & Hilvert (1996) Proc. Natl. Acad. Sci. USA 93, 5043 - 5048
- Kiefer, J., Ebel, N., Schlücker, E., & Leipertz, A. (2010). Characterization of Escherichia coli suspensions using UV/Vis/NIR absorption spectroscopy. Analytical Methods, 9660. doi:10.1039/b9ay00185a
- Kinkhabwala, A., & Guet, C. C. (2008). Uncovering cis regulatory codes using synthetic promoter shuffling. PloS one, 3(4), e2030.
- Krebs in Deutschland 2005/2006. Häufigkeiten und Trends. 7. Auflage, 2010, Robert Koch-Institut (Hrsg) und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e. V. (Hrsg). Berlin.
- Lamparter, T., Michael, N., Mittmann, F., & Esteban, B. (2002). Phytochrome from Agrobacterium tumefaciens has unusual spectral properties and reveals an N-terminal chromophore attachment site. Proceedings of the National Academy of Sciences of the United States of America, 99(18), 11628–33.
- Levskaya, A. et al (2005). Engineering Escherichia coli to see light. Nature, 438(7067), 442.
- Mancinelli, A. (1986). Comparison of spectral properties of phytochromes from different preparations. Plant physiology, 82(4), 956–61.
- Nakasone, Y., Ono, T., Ishii, A., Masuda, S., & Terazima, M. (2007). Transient dimerization and conformational change of a BLUF protein: YcgF. Journal of the American Chemical Society, 129(22), 7028–35.
- Orth, P., & Schnappinger, D. (2000). Structural basis of gene regulation by the tetracycline inducible Tet repressor-operator system. Nature structural biology, 215–219.
- Parkin, D.M., et al., Global cancer statistics, 2002. CA: a cancer journal for clinicians, 2005. 55(2): p. 74-108.
- Rajagopal, S., Key, J. M., Purcell, E. B., Boerema, D. J., & Moffat, K. (2004). Purification and initial characterization of a putative blue light-regulated phosphodiesterase from Escherichia coli. Photochemistry and photobiology, 80(3), 542–7.
- Rizzini, L., Favory, J.-J., Cloix, C., Faggionato, D., O’Hara, A., Kaiserli, E., Baumeister, R., et al. (2011). Perception of UV-B by the Arabidopsis UVR8 protein. Science (New York, N.Y.), 332(6025), 103–6.
- Roux, B., & Walsh, C. T. (1992). p-aminobenzoate synthesis in Escherichia coli: kinetic and mechanistic characterization of the amidotransferase PabA. Biochemistry, 31(30), 6904–10.
- Strickland, D. (2008). Light-activated DNA binding in a designed allosteric protein. Proceedings of the National Academy of Sciences of the United States of America, 105(31), 10709–10714.
- Sinha RP, Häder DP. UV-induced DNA damage and repair: a review. Photochem Photobiol Sci. (2002). 1(4):225-36
- Sambandan DR, Ratner D. (2011). Sunscreens: an overview and update. J Am Acad Dermatol. 2011 Apr;64(4):748-58.
- Tabor, J. J., Levskaya, A., & Voigt, C. A. (2011). Multichromatic Control of Gene Expression in Escherichia coli. Journal of Molecular Biology, 405(2), 315–324.
- Thibodeaux, G., & Cowmeadow, R. (2009). A tetracycline repressor-based mammalian two-hybrid system to detect protein–protein interactions in vivo. Analytical biochemistry, 386(1), 129–131.
- Tschowri, N., & Busse, S. (2009). The BLUF-EAL protein YcgF acts as a direct anti-repressor in a blue-light response of Escherichia coli. Genes & development, 522–534.
- Tschowri, N., Lindenberg, S., & Hengge, R. (2012). Molecular function and potential evolution of the biofilm-modulating blue light-signalling pathway of Escherichia coli. Molecular microbiology.
- Tyagi, A. (2009). Photodynamics of a flavin based blue-light regulated phosphodiesterase protein and its photoreceptor BLUF domain.
- Vainio, H. & Bianchini, F. (2001). IARC Handbooks of Cancer Prevention: Volume 5: Sunscreens. Oxford University Press, USA
- Quinlivan, Eoin P & Roje, Sanja & Basset, Gilles & Shachar-Hill, Yair & Gregory, Jesse F & Hanson, Andrew D. (2003). The folate precursor p-aminobenzoate is reversibly converted to its glucose ester in the plant cytosol. The Journal of biological chemistry, 278.
- van Thor, J. J., Borucki, B., Crielaard, W., Otto, H., Lamparter, T., Hughes, J., Hellingwerf, K. J., et al. (2001). Light-induced proton release and proton uptake reactions in the cyanobacterial phytochrome Cph1. Biochemistry, 40(38), 11460–71.
- Wegkamp A, van Oorschot W, de Vos WM, Smid EJ. (2007 )Characterization of the role of para-aminobenzoic acid biosynthesis in folate production by Lactococcus lactis. Appl Environ Microbiol. Apr;73(8):2673-81.
 "
"