Team:HKUST-Hong Kong/Module/Regulation and control
From 2012.igem.org
| (9 intermediate revisions not shown) | |||
| Line 360: | Line 360: | ||
<div><p align="center"><font size="20">Regulation and Control Module</font></p></div> | <div><p align="center"><font size="20">Regulation and Control Module</font></p></div> | ||
| - | <div><p><a href="https://2012.igem.org/Team:HKUST-Hong_Kong/Module"><<< Back to | + | <div><p><a href="https://2012.igem.org/Team:HKUST-Hong_Kong/Module"><<< Back to Modules</a></p></div> |
| - | <div id="paragraph1" class="bodyParagraphs"> | + | |
| - | + | <div id="paragraph1" class="bodyParagraphs"> | |
| - | + | <div align="left"> | |
| - | + | <h1><p>Overview</p></h1> | |
| - | + | </div> | |
| + | |||
| + | <p>We first introduced a xylose inducible promoter that, when used to express the anti-tumor drug BMP2, can help us control the timing of its expression and secretion. We chose xylose as an inducer because of its induction efficiency, relative scarcity and low absorption rate in the colon.<a href="#_ftn1" name="_ftnref1" title="" id="_ftnref1"> </a>(Yuasa <i>et al.</i>, 1997) Besides the timing regulation, we further introduce a cell growth inhibition device to prevent the overexpression of BMP2. This device is achieved by a balance between a toxin and antitoxin pair, YdcE and YdcD. Under these two regulation systems, our B. hercules can have more reliable and controllable performance.</p> | ||
| + | <p><strong>Objectives:</strong></p> | ||
| + | <p>1. To provide external control over the induction of BMP2 expression, by using a xylose inducible promoter. (Timing regulation.)</p> | ||
| - | + | <p>To prevent overexpression of BMP2. (Dosage regulation.)</p> | |
| - | + | ||
| - | + | ||
| + | </div> | ||
| + | |||
| + | <div id="paragraph2" class="bodyParagraphs"> | ||
| + | <div align="left"> | ||
| + | <h1><p>Design</p></h1> | ||
| + | </div> | ||
| + | |||
| + | <p><strong>Our Module in B. hercules:</strong></p> | ||
| + | |||
| + | <p>1. <em>The inducible promoter. <a href="http://partsregistry.org/Part:BBa_K733002">BBa_K733002</a></em></p> | ||
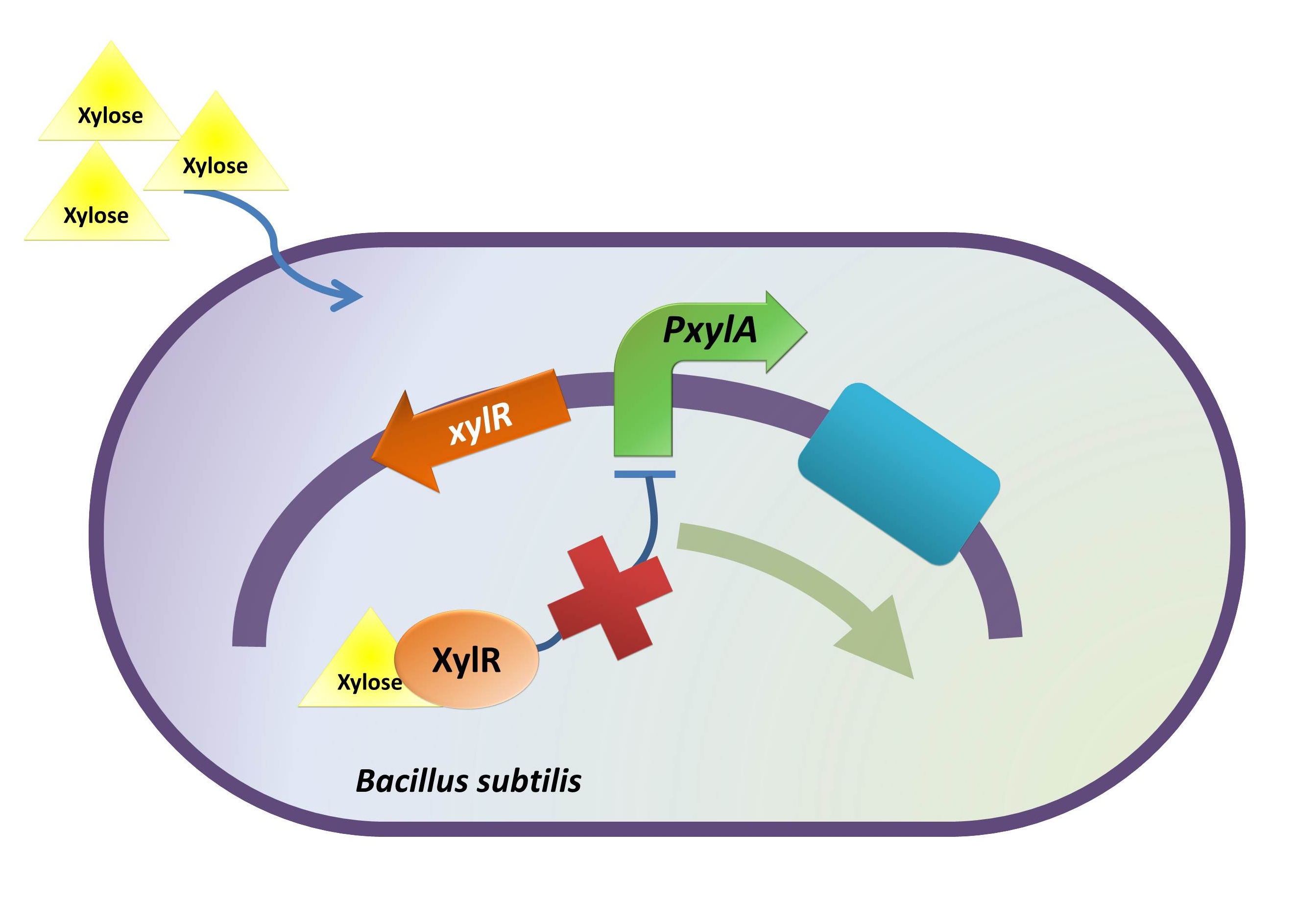
| + | <p>In the consideration of our B. hercules, one of our concerns is that our bacteria may secrete BMP2 before its binding to colon cancer cells. Although BMP2 triggers the apoptosis of colon cancer cell, its better known function is the stimulate of growth and proliferation of normal epithelial cells in digestive tract. <a href="#_ftn2" name="_ftnref2" title="" id="_ftnref2"> </a>(Zhang <i>et al.</i>, 2012) Thus, we intend to introduce a regulatory timing system into our B. hercules by incorporating an inducible promoter into our device.</p> | ||
| + | <p>Admittedly, there are many different induction systems in <em>Bacillus subtilis</em>. However, to achieve the induction when our B. hercules is inside human colon, two conditions need to be taken into consideration: a) the inducer should not normally exist <em>in vivo</em>, but the option should be there to make it available in the human colon; b) the inducer should not vitiate the healthy state of the individual. Furthermore, although high efficiency of the induction is not strictly required, it will still be considerably helpful if could be achieved.</p> | ||
| + | <p>With those concerns in mind we decided on xylose to induce our B. hercules. Xylose, which is the main building block for hemicellulose, can only be found in plants. Largely absorbed in the jejunum before reaching colon, xylose is not typically present in the colon.(Yuasa <i>et al.</i>, 1997) Besides, the absorption rate of xylose in colon is low indicated by Yuasa. Thus, well scheduled diet and medication can prevent the interaction of xylose and B. hercules in an earlier stage of the digestive tract, and induction can therefore be achieved by xylose delivered in enteric capsules or from the anus.</p> | ||
| - | + | <p>Besides its rare existence in the human colon, xylose is an efficient inducer as for <i>PxylA</i> promoter. When ligated with gene <em>bgaB</em>, 200-fold induction was achieved 30 minutes after the induction of xylose.<a href="#_ftn3" name="_ftnref3" title="" id="_ftnref3"> </a> (Kim <i>et al</i>. 1996) | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
</p> | </p> | ||
| - | + | <p align="center"> <img src="https://static.igem.org/mediawiki/2012/c/ce/Xylose_promoter_1.JPG" width="50%" /></p> | |
| - | <p> | + | <p align="center"> <img src="https://static.igem.org/mediawiki/2012/e/eb/Xylose_promoter_2.JPG" width="60%" /></p> |
| - | + | <p align="center"> <img src="https://static.igem.org/mediawiki/2012/7/7b/Xylose_promoter_3.JPG" width="60%" /></p> | |
| - | + | <p>2. <em>The Cell Growth Inhibition Device. <a href="http://partsregistry.org/Part:BBa_K733012">BBa_K733012</a></em></li></em></li> | |
| - | <p | + | <p>Considering the problems caused by the unexpected proliferation of normal colon cells induced by over-dose BMP2, a regulatory system is necessary for the dosage control of BMP2 expression.(Zhang <i>et al.</i>, 2012)</p> |
| + | <p>In order to build this controlling system, we came up with a cell growth inhibition device to manage this task. Understanding that toxin-antitoxin operons exist abundantly in bacteria, we intend to link the expression of BMP2 with a toxin gene. However, the lone presence of the toxin gene is not enough. Stabilization, to a certain extent, is necessary, so that our B. hercules will not die after a low level of BMP2 expression. And this short-term stabilization could be achieved by introducing the corresponding anti-toxin gene of the previous toxin gene. </p> | ||
| + | |||
| + | <p>In order to practically implement the ideas above, a toxin-antitoxin pair – YdcE and YdcD – is used. <i>ydcE</i> encodes an endoribonuclease – EndoA, which causes cell growth inhibition, and is regarded as the "toxin" in this case. On the other hand, <i>ydcD</i> encodes YdcD (EndoAI), which counteracts the effect of EndoA and is regarded as the "anti-toxin" <a href="#_ftn4" name="_ftnref4" title="" id="_ftnref4"> </a>(Pellegrini, O. et al. 2005). By linking <i>ydcE</i> immediately after <i>Bmp2</i> gene, and put <i>ydcD</i> after <i>Ptms</i> promoter, a relatively low efficiency constitutive promoter, EndoA can be expressed simultaneously with the expression of BMP2 under the control of xylose inducible promoter, and cell growth inhibition will not occur until the produced EndoA outweighs the effect of accumulated YdcD (EndoAI).</p> | ||
| + | |||
| + | <p align="center"> | ||
<img src="https://static.igem.org/mediawiki/2012/d/dd/CGIDfunction2..jpg" width="99%" /> | <img src="https://static.igem.org/mediawiki/2012/d/dd/CGIDfunction2..jpg" width="99%" /> | ||
<img src="https://static.igem.org/mediawiki/2012/7/74/CGIDFunction.jpg" width="80%" /> | <img src="https://static.igem.org/mediawiki/2012/7/74/CGIDFunction.jpg" width="80%" /> | ||
| Line 405: | Line 413: | ||
</p> | </p> | ||
| - | <div>< | + | </div> |
| - | <p> | + | |
| - | + | <div id="paragraph3" class="bodyParagraphs"> | |
| + | <div align="left"> | ||
| + | <h1><p>References</p></h1> | ||
| + | </div> | ||
| + | |||
| + | <div id="ftn1"> | ||
<a href="#_ftnref1" name="_ftn1" title="" id="_ftn1"> </a> Yuasa, H., Kuno, C., & Watanabe, J. (1997). Comparative assessment of D-xylose absorption between small intestine and large intestine.. <em>The journal of pharmacy and pharmocology</em>,<em>49</em>, 26-29. </div> | <a href="#_ftnref1" name="_ftn1" title="" id="_ftn1"> </a> Yuasa, H., Kuno, C., & Watanabe, J. (1997). Comparative assessment of D-xylose absorption between small intestine and large intestine.. <em>The journal of pharmacy and pharmocology</em>,<em>49</em>, 26-29. </div> | ||
<div id="ftn2"> | <div id="ftn2"> | ||
| Line 421: | Line 434: | ||
</div> | </div> | ||
| + | <div style="clear:both"> | ||
<p><a href="https://2012.igem.org/Team:HKUST-Hong_Kong/Module/Target_binding">Target Binding Module</a></p> | <p><a href="https://2012.igem.org/Team:HKUST-Hong_Kong/Module/Target_binding">Target Binding Module</a></p> | ||
<p><a href="https://2012.igem.org/Team:HKUST-Hong_Kong/Module/Anti_tumor">Anti-tumor Molecule Secretion Module</a></p> | <p><a href="https://2012.igem.org/Team:HKUST-Hong_Kong/Module/Anti_tumor">Anti-tumor Molecule Secretion Module</a></p> | ||
| + | </div> | ||
<style type="text/css"> | <style type="text/css"> | ||
| Line 439: | Line 454: | ||
-moz-border-radius:10px; | -moz-border-radius:10px; | ||
} | } | ||
| + | |||
| + | #paragraph2{ | ||
| + | background-color:#FFEDFF; | ||
| + | width:955px; | ||
| + | height:auto; | ||
| + | float:left; | ||
| + | padding-bottom:5px; | ||
| + | padding-left:5px; | ||
| + | margin-top:5px; | ||
| + | margin-bottom:5px; | ||
| + | border:3px solid #FFA1FF; | ||
| + | border-radius: 10px; | ||
| + | -moz-border-radius:10px; | ||
| + | } | ||
| + | |||
| + | #paragraph3{ | ||
| + | background-color:#FFFFDD; | ||
| + | width:955px; | ||
| + | height:auto; | ||
| + | float:left; | ||
| + | padding-bottom:5px; | ||
| + | padding-left:5px; | ||
| + | margin-top:5px; | ||
| + | margin-bottom:5px; | ||
| + | border:3px solid #FFFFA1; | ||
| + | border-radius: 10px; | ||
| + | -moz-border-radius:10px; | ||
| + | } | ||
| + | |||
</style> | </style> | ||
<div id="Sitemap"> | <div id="Sitemap"> | ||
Latest revision as of 14:14, 21 October 2012
Regulation and Control Module
Overview
We first introduced a xylose inducible promoter that, when used to express the anti-tumor drug BMP2, can help us control the timing of its expression and secretion. We chose xylose as an inducer because of its induction efficiency, relative scarcity and low absorption rate in the colon. (Yuasa et al., 1997) Besides the timing regulation, we further introduce a cell growth inhibition device to prevent the overexpression of BMP2. This device is achieved by a balance between a toxin and antitoxin pair, YdcE and YdcD. Under these two regulation systems, our B. hercules can have more reliable and controllable performance.
Objectives:
1. To provide external control over the induction of BMP2 expression, by using a xylose inducible promoter. (Timing regulation.)
To prevent overexpression of BMP2. (Dosage regulation.)
Design
Our Module in B. hercules:
1. The inducible promoter. BBa_K733002
In the consideration of our B. hercules, one of our concerns is that our bacteria may secrete BMP2 before its binding to colon cancer cells. Although BMP2 triggers the apoptosis of colon cancer cell, its better known function is the stimulate of growth and proliferation of normal epithelial cells in digestive tract. (Zhang et al., 2012) Thus, we intend to introduce a regulatory timing system into our B. hercules by incorporating an inducible promoter into our device.
Admittedly, there are many different induction systems in Bacillus subtilis. However, to achieve the induction when our B. hercules is inside human colon, two conditions need to be taken into consideration: a) the inducer should not normally exist in vivo, but the option should be there to make it available in the human colon; b) the inducer should not vitiate the healthy state of the individual. Furthermore, although high efficiency of the induction is not strictly required, it will still be considerably helpful if could be achieved.
With those concerns in mind we decided on xylose to induce our B. hercules. Xylose, which is the main building block for hemicellulose, can only be found in plants. Largely absorbed in the jejunum before reaching colon, xylose is not typically present in the colon.(Yuasa et al., 1997) Besides, the absorption rate of xylose in colon is low indicated by Yuasa. Thus, well scheduled diet and medication can prevent the interaction of xylose and B. hercules in an earlier stage of the digestive tract, and induction can therefore be achieved by xylose delivered in enteric capsules or from the anus.
Besides its rare existence in the human colon, xylose is an efficient inducer as for PxylA promoter. When ligated with gene bgaB, 200-fold induction was achieved 30 minutes after the induction of xylose. (Kim et al. 1996)
2. The Cell Growth Inhibition Device. BBa_K733012
Considering the problems caused by the unexpected proliferation of normal colon cells induced by over-dose BMP2, a regulatory system is necessary for the dosage control of BMP2 expression.(Zhang et al., 2012)
In order to build this controlling system, we came up with a cell growth inhibition device to manage this task. Understanding that toxin-antitoxin operons exist abundantly in bacteria, we intend to link the expression of BMP2 with a toxin gene. However, the lone presence of the toxin gene is not enough. Stabilization, to a certain extent, is necessary, so that our B. hercules will not die after a low level of BMP2 expression. And this short-term stabilization could be achieved by introducing the corresponding anti-toxin gene of the previous toxin gene.
In order to practically implement the ideas above, a toxin-antitoxin pair – YdcE and YdcD – is used. ydcE encodes an endoribonuclease – EndoA, which causes cell growth inhibition, and is regarded as the "toxin" in this case. On the other hand, ydcD encodes YdcD (EndoAI), which counteracts the effect of EndoA and is regarded as the "anti-toxin" (Pellegrini, O. et al. 2005). By linking ydcE immediately after Bmp2 gene, and put ydcD after Ptms promoter, a relatively low efficiency constitutive promoter, EndoA can be expressed simultaneously with the expression of BMP2 under the control of xylose inducible promoter, and cell growth inhibition will not occur until the produced EndoA outweighs the effect of accumulated YdcD (EndoAI).


References
Zhang J, Ge Y, Sun L, Cao J, Wu Q, Guo L, Wang Z. Effect of Bone Morphogenetic Protein-2 on Proliferation and Apoptosis of Gastric Cancer Cells. Int J Med Sci 2012; 9(2):184-192.
Project
Wet Lab
Human Practice
 "
"