Team:Cambridge/Lab book/Week 13
From 2012.igem.org
(→Friday (21/09/12)) |
(→Friday (21/09/12)) |
||
| Line 67: | Line 67: | ||
spoVA biobrick (Fast Germination) was succesfully PCR'd out of the pPS3393 minipreps. Lanes 2-8 represent pPS3393 minipreps. The band at around 600bp was extracted. | spoVA biobrick (Fast Germination) was succesfully PCR'd out of the pPS3393 minipreps. Lanes 2-8 represent pPS3393 minipreps. The band at around 600bp was extracted. | ||
| - | [[File:SPOBB gel. | + | [[File:SPOBB gel.JPG]] |
===Saturday (22/09/12)=== | ===Saturday (22/09/12)=== | ||
Revision as of 03:23, 27 September 2012
| Week: | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|
Contents |
Monday (17/09/12)
Ratiometrica - Lux
Testing of the 2.0 construct:
Numbers 33,35,36 have correct sequence as of being sent to us. However they were not visibly luminescent. *34 was.
Overnight cultures of each of the 6 culture lines sent were set up. Additionally, inducing plates were streaked, including the 2010 lux biobrick as a luminescence control and pjs130 as an iptg induction control.
Primers ordered to ligate 2.0 construct into pjs130 for insertion into bacillus.
Tuesday (18/09/12)
Ratiometrica - Lux
None of restreaked plates visibly luminescent, aside from *34 (faint), even under photon counting camera. Pjs130 not green, so iptg induction potentially didn't work. LuxBB induced and luminescent.
Cultures induced with 1mM iptg or 3mM arabinose for luxBB, imaged after 3 hrs. Some extremely faint luminescence for 33,34,38 on photon counting camera.
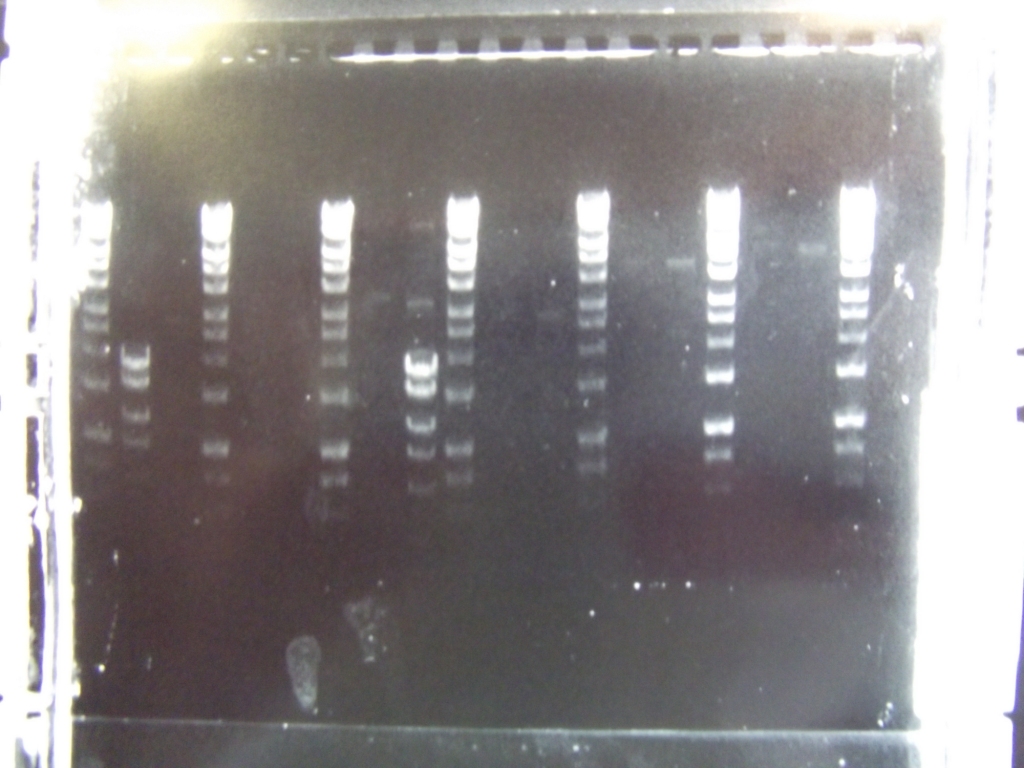
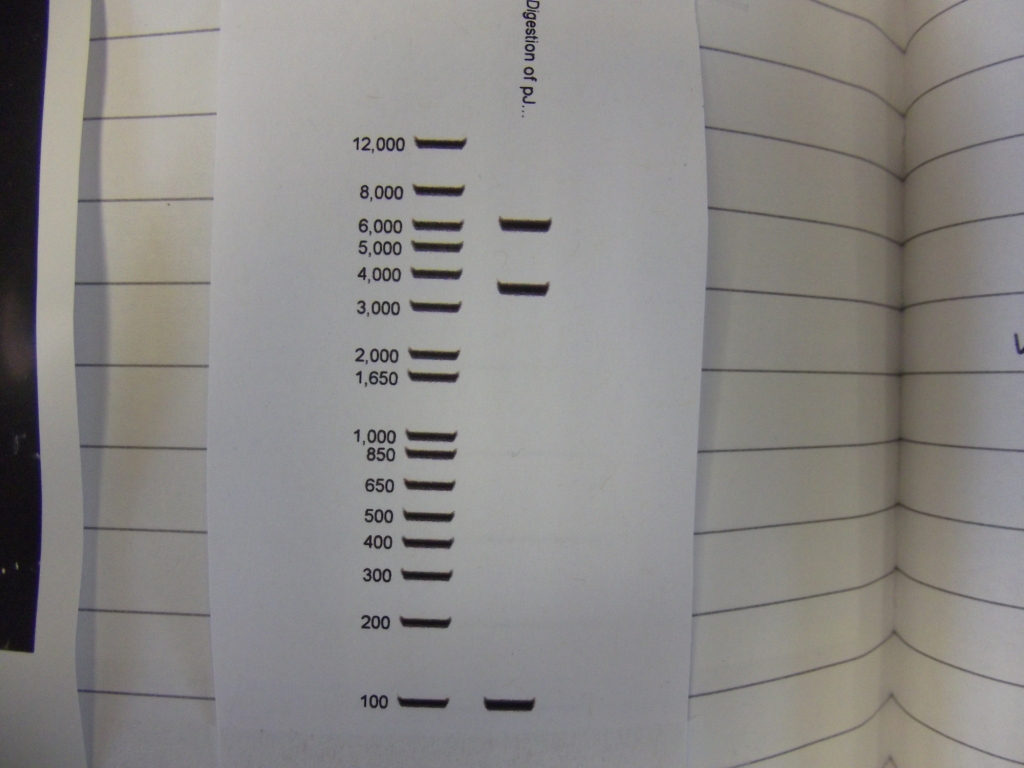
Cultures miniprepped and restriction digests carried out w/spa1. Results are shown. Results indicate problem with the miniprep and/or damage to the construct - expected bands at 6625, 2838, 1876 and 53 bp.
Wednesday (19/09/12)
Ratiometrica - Lux
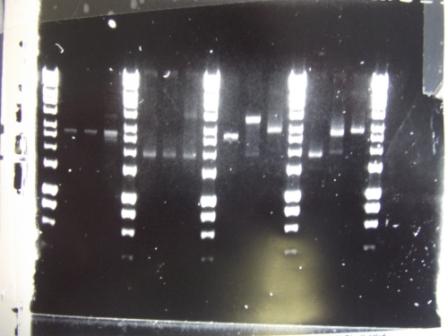
Digest of 2.0 miniprep with different enzyme, hindIII, carried out. 15ul template DNA used in a 20ul reaction. Again, results were odd. Cut and uncut minipreps ran.Cultures of ratiometrica (fluorescent) were miniprepped to be transformed into bacillus. A restriction digest was performed on these minipreps, and produced bands of the correct size.
Thursday (20/09/12)
Ratiometrica - Lux
Transformation of fluorescent ratiometric construct into bacillus was unsuccessful
Transformations of XL1-blue cells with resuspended construct plasmid from DNA 2.0 yielded orange colonies, with some white, and some showing sectoring, indicating insert loss.
Plates all appear to be luminescent. A random selection of colonies was picked and patched in order to investigate whether luminescence cosegregated with orange colour.
Friday (21/09/12)
Initial pick and patch experiments showed cosegregation, but we did not have immediate access to a photon-counting camera or a DSLR capable of long exposures, so we did not obtain images. Representative colonies were streaked out onto a fresh plate to be imaged later.
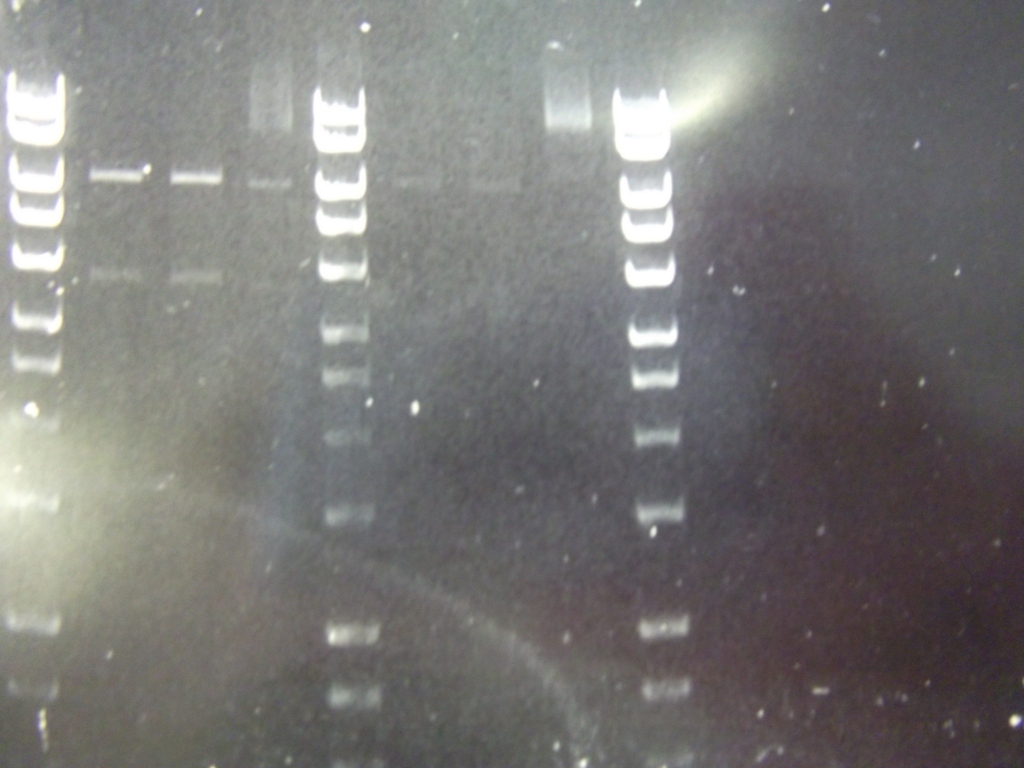
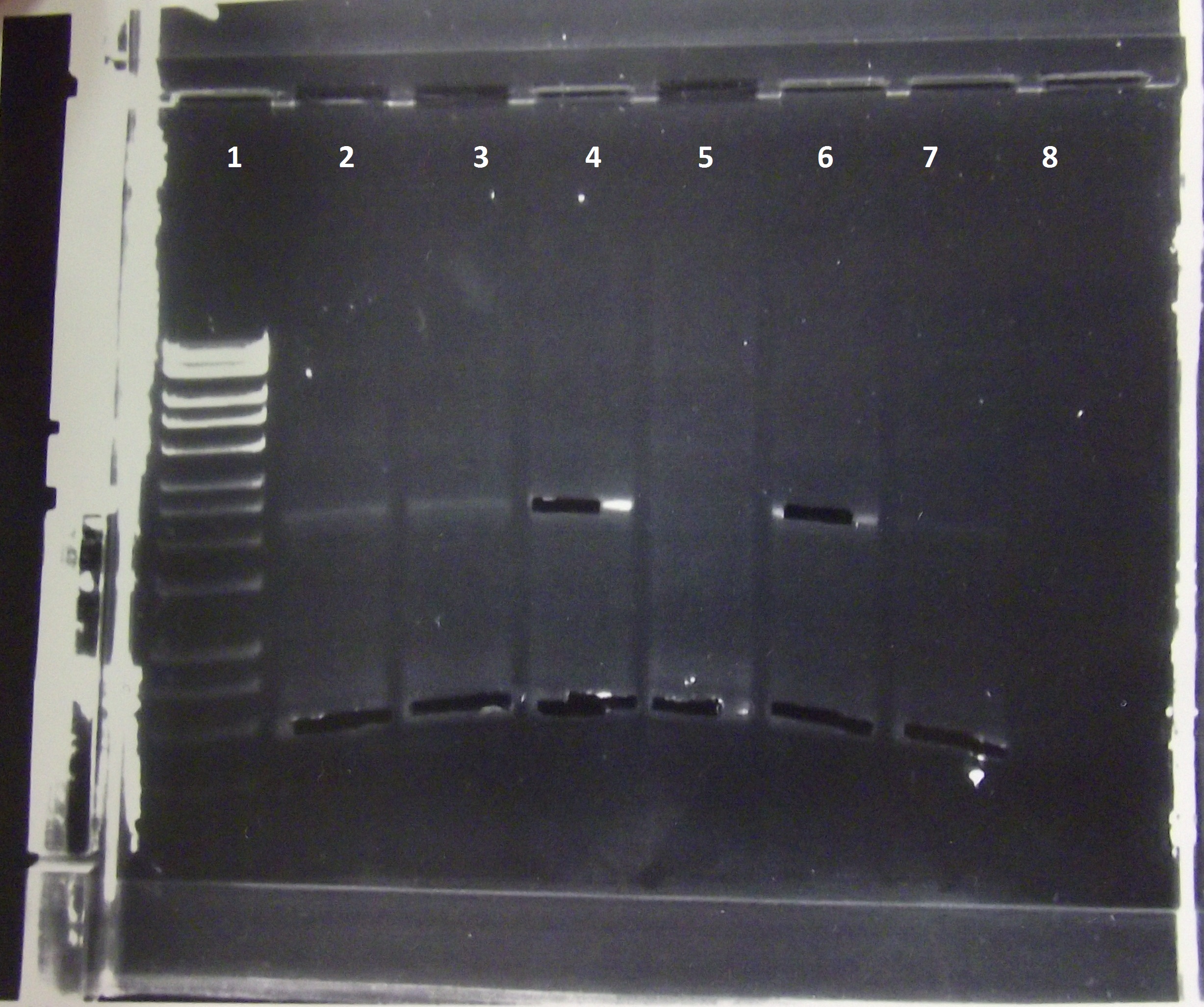
spoVA biobrick (Fast Germination) was succesfully PCR'd out of the pPS3393 minipreps. Lanes 2-8 represent pPS3393 minipreps. The band at around 600bp was extracted.
Saturday (22/09/12)
A PCR was run to amplify several fragments, including:
- The ratiometric luciferase construct, to insert into;
- pSB3C5, a low copy number vector
- Oli's Mg riboswitch construct
All fragments were obtained.
Sunday (23/09/12)
Fragments for the fluoride riboswitch, the spoVA swap biobrick and the insertion of the 2.0 construct into pSB3C5 were all digested for two hours in 50 ul reactions, as was the pSB1C3 backbone.
- 5ul NEB2
- 1 ul EcoRI
- 1 ul PstI
- 0.5 ul BSA
- 10 ul DNA
- 32.5 ul HPLC H20
Enzyme was heat-inactivated for 20 minutes at 65 C
for a 6:1 insert:vector ratio (recommended by Knight on openwetware) 2.5 ul of vector digest was ligated to 15 ul insert in all cases, except the luciferase construct. In this case the insert is bigger than the vector, so the ratio was reversed.
- 3ul vector
- 18ul insert
- 2.5ul 10X T4 ligase buffer
- 1.25 ul T4 ligase
Ligation left overnight at 16C, as recommended by Knight.
 "
"