|
|
August 1st, 2012
- lgt-pUC19 *4
- GFP-pUC19 *4
- lgt-GFP-pUC19
Digestion identification
- Result: GFP-pUC19(3) successful
- Correct sequence
Colony Picking
- lac-pUC19, lgt-GFP-pUC19
August 2nd, 2012
Digestion identification
Reconstructed plasmid
System
| Enzyme
| 1μl
|
| 10×FD Buffer or FD Green buffer | 2μl
|
| DNA | ≤1μg
|
| Sterile water | up to 20μl
|
August 3rd, 2012
Digestion identification
System
| Enzyme
| 1μl
|
| 10×FD Buffer or FD Green buffer | 2μl
|
| DNA | ≤1μg
|
| Sterile water | up to 20μl
|
August 4th, 2012
Ligation
- LLG with pUC
- LLG with pRSF
Ligation System
| 10X T4 DNA Ligase Buffer
| 2 μl
|
| Vector DNA
| 0.025 pmol
|
| Insert DNA
| 0.076 pmol
|
| T4 DNA Ligase
| 1μl
|
| Nuclease-free water
| to 20 μl
|
August 5th, 2012
PCR
- fl3 with rbsABCE
- fl3 with rbsABDE
System
- kod enzyme
Transformation
August 6th, 2012
Digestion
- Llg with Ecor1
- Llg with prst1
System
| Ecor1,prst1
| 1μl
|
| 10×FD Buffer or FD Green buffer | 2μl
|
| DNA | ≤1μg
|
| Sterile water | up to 20μl
|
Colony Picking
August 7th, 2012
 Fig8.7 Digestion identification Digestion identification
System
| Ecor1
| 1μl
|
| 10×FD Buffer or FD Green buffer | 2μl
|
| DNA | ≤1μg
|
| Sterile water | up to 20μl
|
August 8th, 2012
PCR
- VioC with FL3
- Reaction temperature:
Three-step method:
Predenature
94℃,2min
Denature,annealing and extension
98℃ 10sec
{
(56)℃ 30sec
68℃ 30sec/kb
}
× 34 cycles
4℃
Transformation
August 9th, 2012
Colony Picking
August 10th, 2012
PCR
- fl3 with vio A*2
- with Vio B
Transformation
- MFL3-vioA1
- MFL3-vioA2
- MFL3-vioB1
August 11th, 2012
Ligation
- dsbAss-mFL3-F with lgt-R
- 1mFL3-SH3d with SH3-SP-R
- dsbASS-PD2L-mFL3-F with lgt-R
- dsbASS-PD2d-F with PD2d-SP-R
- 1mFL3-GBDd-F with GBDd-SP-R
system
- 10X T4 DNA Ligase Buffer 2 μl
- Vector DNA 0.025 pmol
- Insert DNA 0.076 pmol
- T4 DNA Ligase 1μl
- Nuclease-free water to 20 μl
Colon picking
- MFL3-vioA1
- MFL3-vioA2
- MFL3-vioB1
August 12th, 2012
- MFL3-vioA1
- MFL3-vioA2
- MFL3-vioB1
PCR identification
- MFL3-vioA1
- MFL3-vioA2
- MFL3-vioB1
August 13th, 2012
PCR
Ligation
- Vio A with fl3
- Vio B with fl3
- Vio D with fl3
- Vio E with fl3
August 14th, 2012
Confocal imaging
fluroescent protein expression with concentration gradient test
- 0.2% Ara induction
- 2% Ara induction
Transformation
- fl3-VioA
- fl3-VioB
- fl3-VioD
- fl3-VioE
August 15th, 2012
Double antibody screening
- AMP 50ug/mL Ara 0%
- AMP 50ug/mL Ara 0.02 %
- AMP 0ug/mL Ara 0.02 %
Colon picking
- fl3-VioA
- fl3-VioB
- fl3-VioD
- fl3-VioE
August 16th, 2012
PCR identification
control group of fatty acid synthesis acceleration system
- rbs-TestA
- rbs-FabZ
- rbs-FabG
Reaction temperature:
Three-step method:
Predenature
94℃,2min
Denature,annealing and extension
98℃ 10sec
{
(56)℃ 30sec
68℃ 30sec/kb
}
× 34 cycles
4℃
August 17th, 2012
 Fig8.17 Digestion results Digestion identification
control group of fatty acid synthesis acceleration system
Restriction enzyme: EcoRI, XbaI
system
- Enzyme 1μl
- 10×FD Buffer or FD Green buffer 2μl
- DNA ≤1μg
- Sterile water up to 20μl
August 18th, 2012
Ligation
fatty acid synthesis acceleration system
- M-TestA:rbs-dsbAss-FL3-lgt-FL3-SH3domain-FL3 with TestA
- M-FabG:rbs-dsbAss-PDZligand-FL3-lgt-FL3-SH3ligand-FL3 with FabG
- M-FabI:rbs-dsbAss-PDZdomain-FL3-lgt-FL3-GBDdomain-FL3 with Fab I
- M-FabZ:rbs-dsbAss- FL3-lgt-FL3-GBDligand-FL3 with FabZ
system
- 10X T4 DNA Ligase Buffer 2 μl
- Vector DNA 0.025 pmol
- Insert DNA 0.076 pmol
- T4 DNA Ligase 1μl
- Nuclease-free water to 20 μl
August 19th, 2012
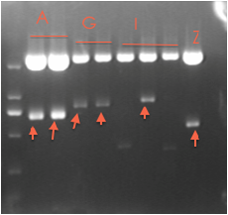 Fig8.19 digestion results Digestion identification for ligation production
fatty acid synthesis acceleration system
- Restriction enzyme: EcoRI, XbaI
system
- Enzyme 1μl
- 10×FD Buffer or FD Green buffer 2μl
- DNA ≤1μg
- Sterile water up to 20μl
August 20th, 2012
 Fig8.20 digestion results Digestion identification
control group of fatty acid synthesis acceleration system
Restriction enzyme: EcoRI, XbaI
system
- Enzyme 1μl
- 10×FD Buffer or FD Green buffer 2μl
- DNA ≤1μg
- Sterile water up to 20μl
August 21st, 2012
 Fig8.21 digestion results Digestion identification
Restriction enzyme: EcoRI, XbaI
system
- Enzyme 1μl
- 10×FD Buffer or FD Green buffer 2μl
- DNA ≤1μg
- Sterile water up to 20μl
August 22nd, 2012
Ligation
- M1 with EGFP1
- M2 with EGFP2
- M3 with EGFP1
- M4 with EGFP2
System: 5ul solutionI + 0.5ul fusion membrane + 4.5ul according EGFP
August 23rd, 2012
transformation
System: 2ul ligation products with 30ul competent cells
Colon picking
- M1 with EGFP1 *4
- M2 with EGFP2 *4
- M3 with EGFP1 *4
- M4 with EGFP2 *4
August 24th, 2012
Confocal imaging
fluroescent protein expression
PCR identification
- rbsvioB-pACYC
- rbsvioA-pET
Three-step method:
Predenature
94℃,2min
Denature,annealing and extension
98℃ 10sec
{
(58)℃ 30sec
68℃ 30sec/kb
}
× 36 cycles
4℃
August 25th, 2012
 Fig7.4.2 digestion result Digestion identification
Restriction enzyme: EcoRI, XbaI
system
- Enzyme 1μl
- 10×FD Buffer or FD Green buffer 2μl
- DNA ≤1μg
- Sterile water up to 20μl
August 26th, 2012
PCR identification
Three-step method:
Predenature
94℃,2min
Denature,annealing and extension
98℃ 10sec
{
(56)℃ 30sec
68℃ 30sec/kb
}
× 32 cycles
4℃
August 27th, 2012
Digestion identification
Restriction enzyme: EcoRI, XbaI
System
| Enzyme
| 1μl
|
| 10×FD Buffer or FD Green buffer | 2μl
|
| DNA | ≤1μg
|
| Sterile water | up to 20μl
|
August 28th, 2012
Transformation
- rbsvioA-pET
- M1-EGFP1
- M2-EGFP2
August 29th, 2012
colon picking
- rbsvioA-pET
- M1-EGFP1
- M2-EGFP2
August 30th, 2012
Ligation
Transformation
system
- 10X T4 DNA Ligase Buffer 2 μl
- Vector DNA 0.025 pmol
- Insert DNA 0.076 pmol
- T4 DNA Ligase 1μl
- Nuclease-free water to 20 μl
August 31st, 2012
Confocal imaging
fluroescent protein expression
Colon picking
|