|
|
July 1st, 2012
- dsbASS, lactamase, lgt, GFP, FL3, S1P, S2P, pETDuet-1, pACYLDuet-1, pRSFDuet-1, PBAD-1
- 3 tubes each, 11*3=33 tubes altogether.
- pBAD, S1P, S2P needs to be extracted again.
Digestion Identification
- Restriction enzyme: EcoRI, PstI
- Only lactamase 1-2, GFP1-2 have correct bands
- Pick colonies of dsbASS, lgt and FL3 again and incubate in 2ml EP tubes(*10).
July 2nd, 2012
Tailing and Colony Picking
- pET, pRSF, pACYC, pBAD, vioD, vioE
- Each pick 4 colonies.
PCR
- lgt*7, dsb*7, FL3*7, S1P*5, S2P*3
- vioA, vioB, vioC, vioD, vioE
- vioABCE
Incubation
- S1P-1, S1P-2, S2P-1, S2P-3, dsbASS-6, dsbASS-1, FL3-6, FL3-3, FL3-7, 10 tubes altogether
- 10ul into 5ml EP tube, incubated in 37℃.
Transformation
- Transform PDZ(5.2ng/ul), GBD(3.3ng/ul) and SH3(7.7ng/ul) 1ul each into 30ul competent cells.
- Transform vioABCE and vioABDE(Kn resistant) 0.5ul each into 50ul competent cells.
July 3rd, 2012
PCR
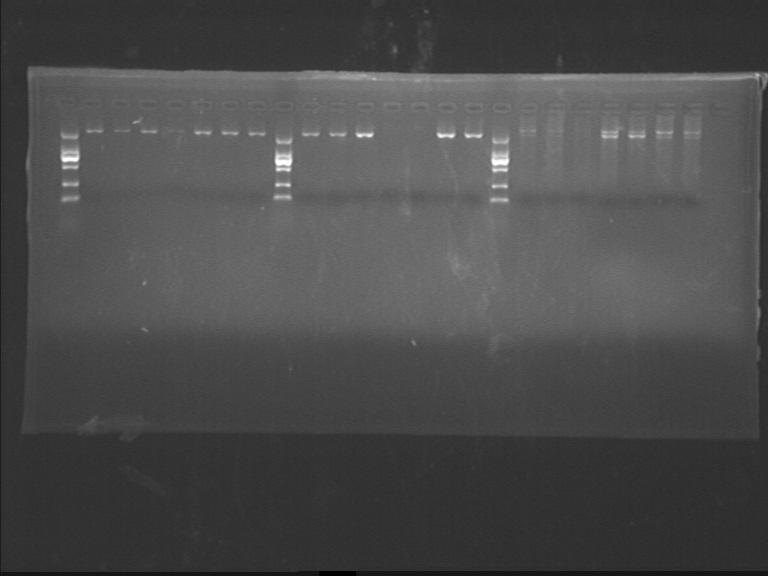 Fig7.3 Diegestion results - dsbASS(bacteria), site1 and site2(plasmid)
Incubation
- Pick colonies from the plates (PDZ, GBD, SH3, vioABCE and vioABDE). Incubated from 9:00am for 24h.
- Incubate pACYC and pRSF(1ul into 5ml)
- pET 1-4, pBA 1-4, vioD 1-3, vioE 1-4
- FL3 1-7, S1P 1-2, S2P 2-3, vioABCE 1, vioABCE 2
Digestion identification
- Restriction enzyme: EcoRI, PstI
July 4th, 2012
- pACYC, pRSF
PCR and Gel Extraction
- dsbAss, lgt, FL3 (3-step method)
- Only FL3 has band.
Colony Picking
- vioABCE, vioABDE, GFP, lactamase
July 5th, 2012
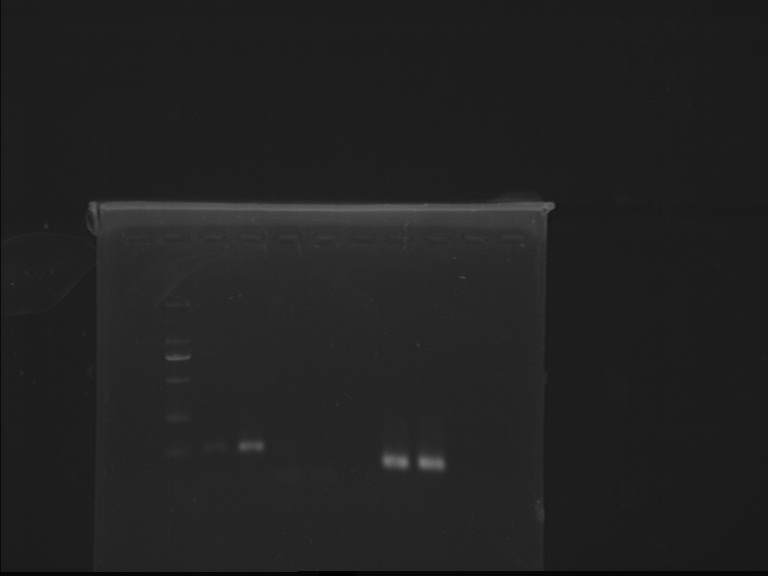 Fig7.5 PCR and tailing results Tailing
- FL3
PCR
- dsbASS, lgt
- (KOD, 2-step method)
Connection and Transformation
- Connect FL3 with T vector. (Solution I system)
- 2ul connection product was transformed into 50ul DH5a, coated 2 plates(Amp)
July 6th, 2012
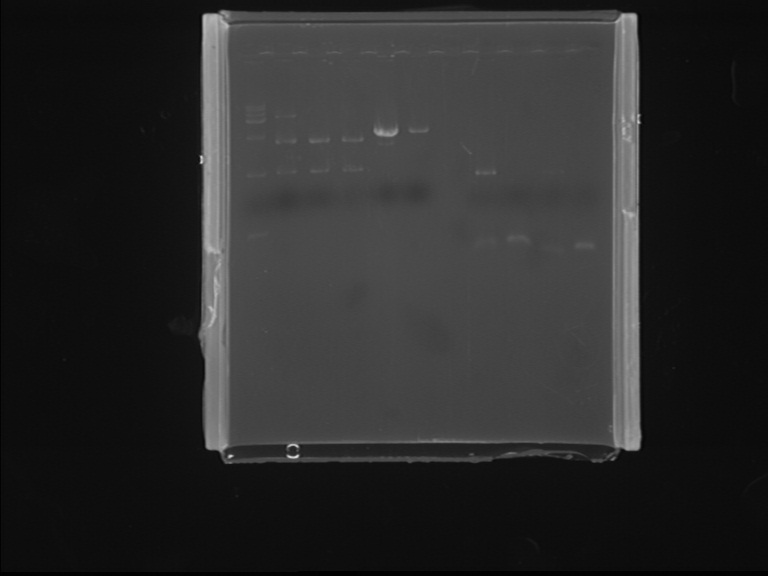 Fig7.6 PCR and digestion results Digestion identification
- lgt and dsbASS
PCR
- lgt and dsbASS
July 7th, 2012
Colony Picking
- Plates: FL3-T-1, FL3-T-2(coated on July 5th)
- Pick 3 colonies from each plates
Incubate
- Incubated at 37℃ from 9:30am
July 8th, 2012
Transformation
- GWPT1 and GVPT1, each 1ul, were transformed into 50ul competent cells, coated 2 plates(Kn)
- GW334, JD504and JD505, each 2ul, were transformed into 30ul competent, coated 3 plates(Amp)
- FL3-1 and FL3-2, 3 tubes each
Digestion identification
- Restriction enzyme: EcoRI, PstI
- No objective sequences
July 9th, 2012
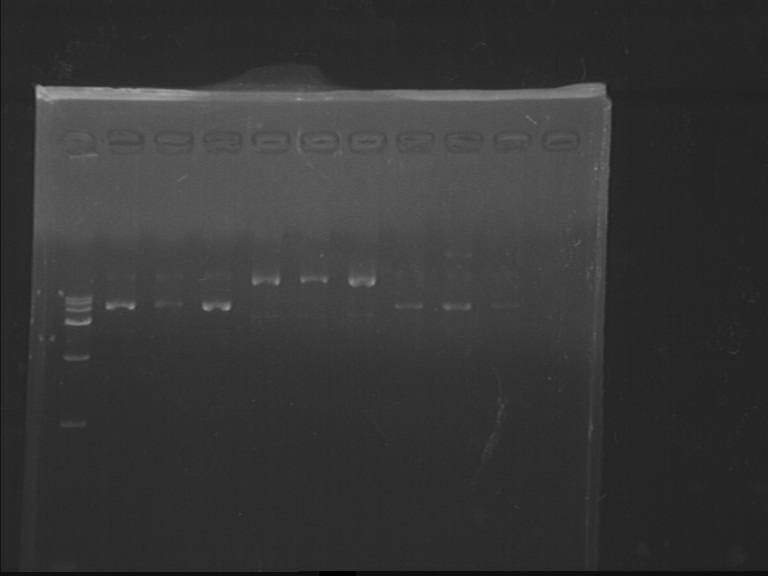 Fig7.9 pACYC, pET and pRSF plasmids Colony Picking
- VVD, JD504, JD505, GW334 in the morning, pick 2 colonies from each plate.
- lgt in the afternoon, pick 3 colonies.
- Incubate at 240rpm for 20h.
- pACYC-1(26.6ng/ul), pACYC-2(17.7ng/ul), pACYC-3(27.0ng/ul)
- pET-1(26.1ng/ul), pET-2(16.8ng/ul), pET-3(28.7ng/ul)
- pRSF-1(17.1ng/ul), pRSF-2(30.2ng/ul), pRSF-3(9.9ng/ul)
- The size of DNA fragments may be incorrect.
July 10th, 2012
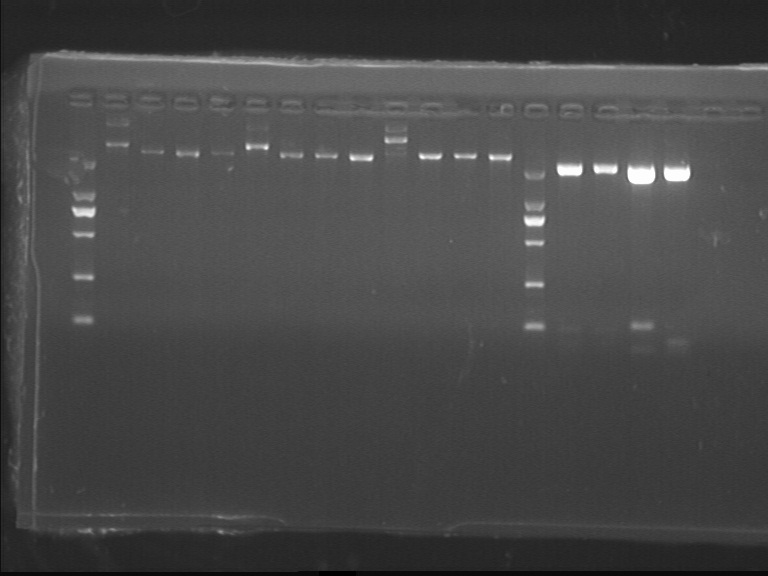 Fig7.10.1 Digestion identification of vectors and lgt-T
- lgt1-1(71.6ng/ul), lgt1-2(48.2ng/ul), lgt2-1(183.2ng/ul), lgt2-2(131.3ng/ul)
- Each has 2 bands and high brightness.
Digestion Identification
- pSRF, pET and pACYC cut by HpaI
- lgt-T cut by EcoRI and PstI
PCR and Gel Extraction
- FL3(27.3ng/ul), dsbASS(18ng/ul), lgt(26.8ng/ul)
July 11th, 2012
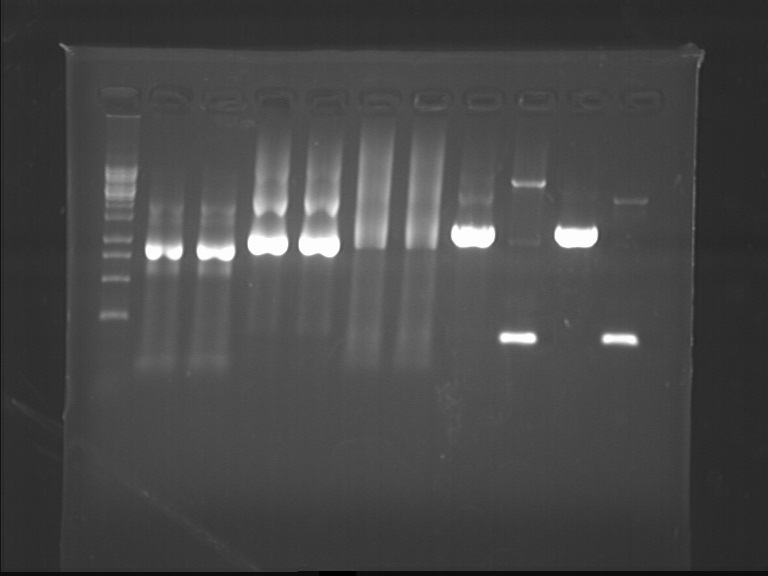 Fig7.11 Connection products Tailing
- dsbA11(91.7ng/ul), dsbA22(91.7ng/ul), FL311(52.6ng/ul), FL322(59.7ng/ul), lgt11(8.7ng/ul), lgt22-1(21.9ng/ul), lgt22-2(41.4ng/ul)
- The size of the bands was correct
Connection
- System: 5ul solutionI + 0/5ul T-vector + 4.5ul DNA
- dsbASS-lac, FL3-lgt, lgt-site1, lgt-site2
- Begin at 16:00pm
- pACYC-2(88.2ng/ul), pACYC-3(127.1ng/ul), pACYC-4(108.4ng/ul)
- pET-1(80.0ng/ul), pET-2(138.2ng/ul), pET-4(123.9ng/ul)
- pRSF-3(109.4ng/ul), pRSF-4(107.0ng/ul)
Digestion Identification
- Restriction enzyme: HpaI
- pRSF-3&4, pACYC-2&3&4, pET-1&2&4, siteI(pBAD),site2(2-1E)
July 12th, 2012
PCR
- Bacteria solution: dsb-1(1-8), dsb-2(1-8), lgt-1(1-8), lgt-2(1-8), 0.5ul each
- KOD, 3-step method
- Successful PCR products: dsb*3, lgt*3
- Incubate the correct bacteria(dsb*3, lgt*3) overnight
July 13th, 2012
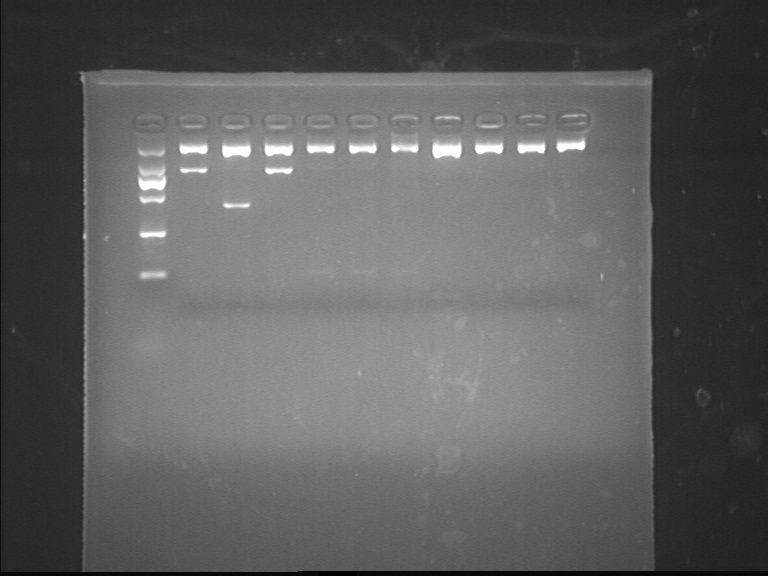 Fig7.13.2 Digestion Identification PCR
- FL3
- KOD, 3-step method
Digestion Identification
- Restriction enzyme: EcoRI, SpeI
- lgt, dsbASS, FL3
July 14th, 2012
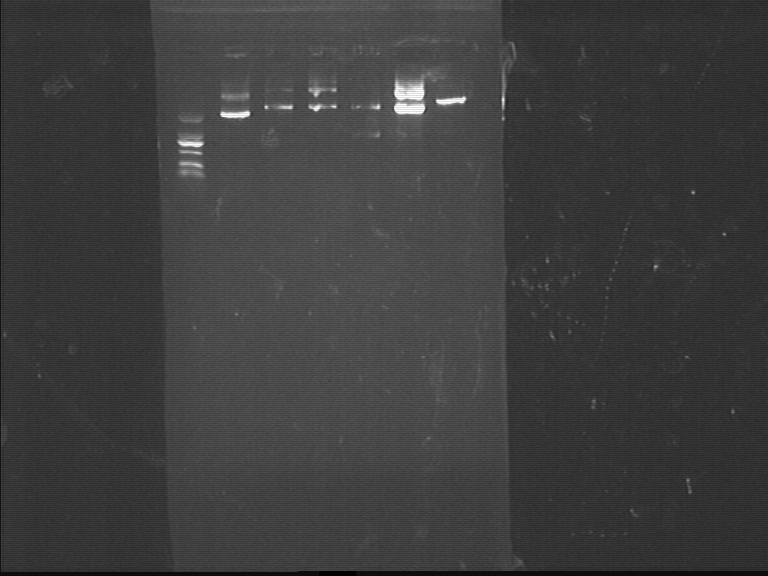 Fig7.14 Digestion identification Purification
- PCR products of FL3
Digestion Identification
- Restriction enzyme: EcoRI, XbaI
- lac, lgt, GFP
- dsbASS*3, GFP*2, lac*2, lgt*3
- Preserve the strains.
Connection
- FL3-GFP, FL3-lgt
- System: 5ul solutionI + 0.5ul GFP/LGT + 4.5ul FL3
July 15th, 2012
 Fig7.15.1 PCR results(FL3-GFP)  Fig7.15.2 PCR results(FL3-lgt) Colony Picking
- Four plates: FL3-GFP*2, FL3-lgt*2
- Pick 15 colonies from each plate
PCR
- Bacteria solution(FL3-GFP, FL3-lgt)
- Result: no bands
July 16th, 2012
Digestion Identification
- FL3 cut by EcoRI/SpeI
- GFP and lgt cut by EcoRI/XbaI
- System: 20ul (Takara)
- Denatured under 65℃ first
July 17th, 2012
Connection
- FL3-GFP, FL3-lgt
July 18th, 2012
Colony Picking
- lgt2(16 colonies)
- FL3-GFP, FL3-lgt
- Begin at 10:00am
PCR
- lgt
- KOD, 3-step method
 Fig7.18.2 Digestion identification Digestion Identification
- Restriction enzyme: EcoRI/PstI
- Connection products: FL3-GFP, FL3-lgt
- System: 20ul
July 19th, 2012
 Fig7.19 Digestion Identification of lgt Plasimid Extraction and Digestion Identification
- lgt-8(85.3ng/ul), lgt-9(90.4ng/ul), lgt-11(110.5ng/ul)
- Restriction enzymes: E/P, E/X
- Result: unsuccessful reverse connection
Connection
- Digestion: EcoRI,PstI, begin at 13:00
- Denatured at 65℃
- Connection system: pMD18-T 0.5ul + dsbASS 1ul + Solution I 5ul + ddH2O
Transformation
- dsbASS-T, GFP-T
July 20th, 2012
- pET-1: no bands
- pET-2: incorrect sequence
- vioABDE: no bands
Colony Picking
- S1S2pET, S1S2pRSF
Digestion
- S1S2pET and S1S2pRSF cut by BsgGI/XhoI(11:00)
July 21st, 2012
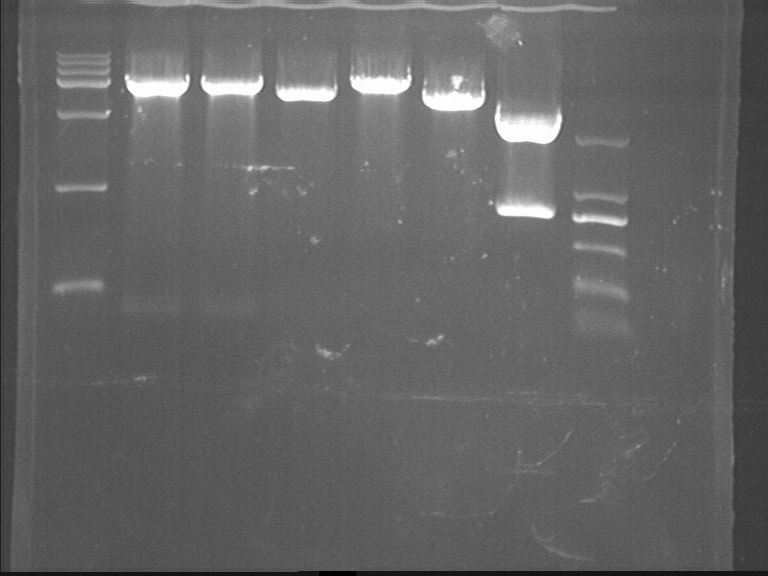 Fig7.21.1 Digestion results
- pET: no bacteria growing
- pACYC; correct
Digestion
- S1S2pACYC cut by BsgGI/XhoI
- S1S2pACYC, S1S2pRSF, S1pET and GFP-T cut by EcoRI/PstI (recycled)
PCR
- site1-F and site1-R: correct
- site2-F and site2-R: correct
Connection
- GFP 2ul + Plasmid 1ul + Solution 3ul
- site2 0.5ul + S1pET 2.5ul + Solution 3ul
- For 4 hours
Transform
- 50ul Top10 competent cells + 2.5ul connection product
July 22nd, 2012
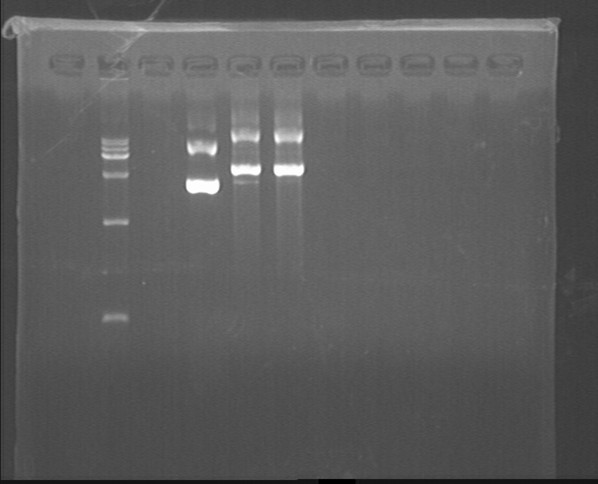 Fig7.22 Digestion results
Digestion
- dsbASS-T, GFP-T-1, GFP-T-2
PCR
- to test whether dsbASS and T vector have been connected
- Taq, 1ul template
Transformation
- Connection product: lgt-T
July 23rd, 2012
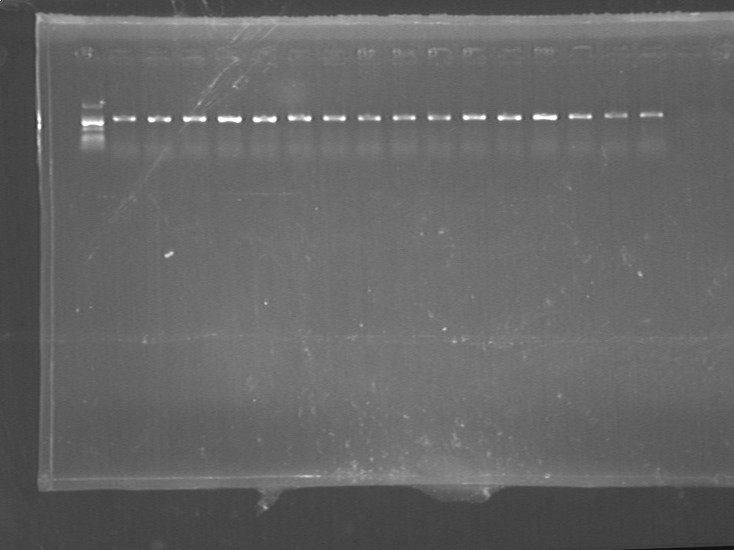 Fig7.23.1 PCR results(lgt) PCR
- a. identification of lgt
- b. identification of GFP-Plasmid
July 24th, 2012
 Fig7.24.1 Digestion results(E/X,E/P) Digestion
- Restriction enzymesL EcoRI/PstI, EcoRI/XbaI
- Plasmid: lgt
- Result: E/P positive, E/X negative
Construction
- lac, lgt-2: EcoRI/SpeI
- lgt-1, GFP: EcoRI/XbaI
- Gel extraction: lac(6ng/ul),lgt2(12.4ng/ul), lgt1(30.8ng/ul, GFP(4.2ng/ul)
- Connection(6h): 1.5ul GFP-T + 3.5ul lgt2 + 5ul Solution I, 0.5ul lgt1 + 4.5ul lac + 5ul Solution I
PCR
- Identification of dsbASS-T
July 25th, 2012
PCR
- L-L, L-G
- Primers: (lac-F,lgt-R), (lgt-F,GFP-R)
- Taq, annealing temperature 56℃
- Result: correct bands
Colony Picking
- Pick corresponding L-L, L-G colonies and incubate for 12-24h
Purification
- L-L(30.0ng/ul), L-G(25.5ng/ul)
Digestion
- dsbASS cut by EcoRI/PstI
- GFP-pRSF cut by EcoRI/XbaI
July 26th, 2012
Plasmid Extraction and Digestion
- Plasmid: L-L, L-G
- L-L cut by EcoRI/PstI(2h)
- L-G cut by EcoRI/XbaI(2h)
Connection
- System: 4.5ul lac + 0.5ul lgt-GFP + 5ul Solution I
July 27th, 2012
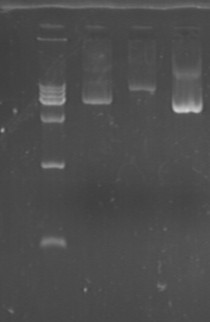 Fig7.27.1 Plasmid(S1S2@pACYC,pET,pRSF) 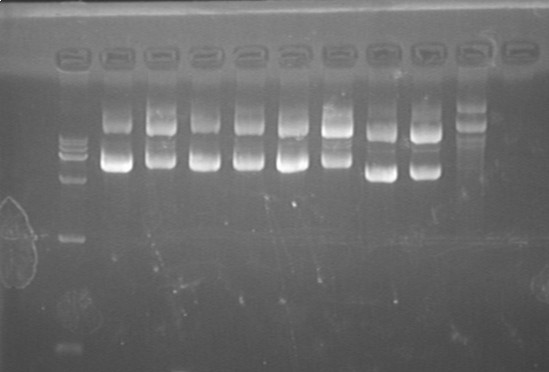 Fig7.27.2 Plasmid(L-L1-6,L-G1-3)
- S1S2 at pACYC, pET, pRSF
- L-L 1,2,3,4,5,6
- L-G 1,2,3
July 28th, 2012
PCR
July 29th, 2012
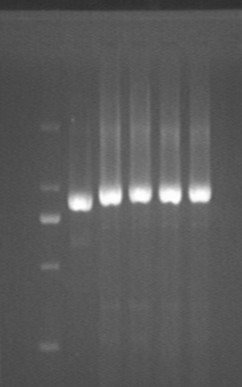 Fig7.29.1 Digestion results
- pRSF *2
Digestion
- lac-dsbASS
- lac *4
Purification
- PCR product: dsb-F + overlap-R
July 30th, 2012
Connection
- a.pACYC-S1GFP, b.pET-S1GFP, c.pRSF-S1GFP,d.pUC19-GFP(FL3),e.pUC19-lgt(FL3),f.pACYC-S2GFP
PCR
- rbs-dsb-lac *8
- FL3-GFP *4 (GFP-T)
- FL3-lgt *4 (lgt-Plasmid)
Transformation
- S1S2pET-S1GFP, S1S2pRSF-S1GFP, FL3-GFP
- FL3-lgt, pUC19(1.5ul), dsb-lac
July 31st, 2012
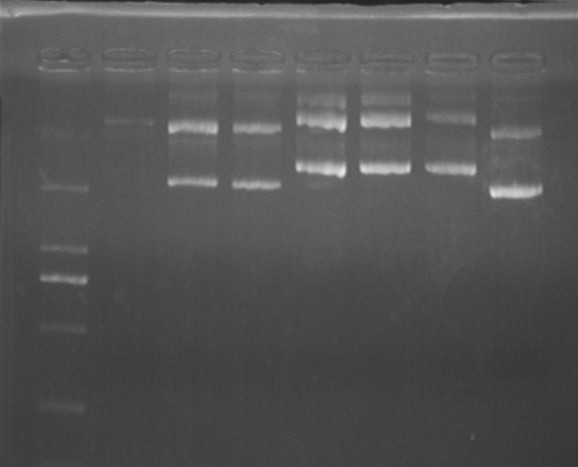 Fig7.31 Digestion identification of the plasmids
- lgt *2
- lgt-GFP *4
Digestion Identification
- Result: unsuccessful conncetion
Transformation
- lac-pUC19
- lgt-GFP-pUC19
|