Team:Columbia-Cooper-NYC/Data and Conclusions
From 2012.igem.org
Data and Conclusions
Liquid Media
As mentioned in the Overview, Acidithiobacillus Ferrooxidans have the ability to oxidize iron (II) to iron (III) and the iron (III) can go through a redox reaction with copper to solubilize copper, which is the goal. But this mechanism only works in aerobic conditions. Therefore, to properly induce mass transfer of oxygen from the media to the bacteria, flasks containing the liquid media and bacteria are placed in a shaker at 220 rpm and room temperature. The copper used in these experiments are 2 cm x 2 cm pieces, 5 mils thick (computer paper thickness), and 99.9% pure. Individual copper pieces were placed in flasks containing 100 mL of liquid media and either bacteria, no bacteria, or iron (III) sulfate hydrate. These flasks were left in the shaker for four days and copper mass measurements were taken daily in triplicate. The copper piece were massed after cleaning with deionized water and 70% ethanol followed by returning them back to their respective flasks. Note that A. Ferrooxidans undergo a 20 hour lag phase before growth can start.
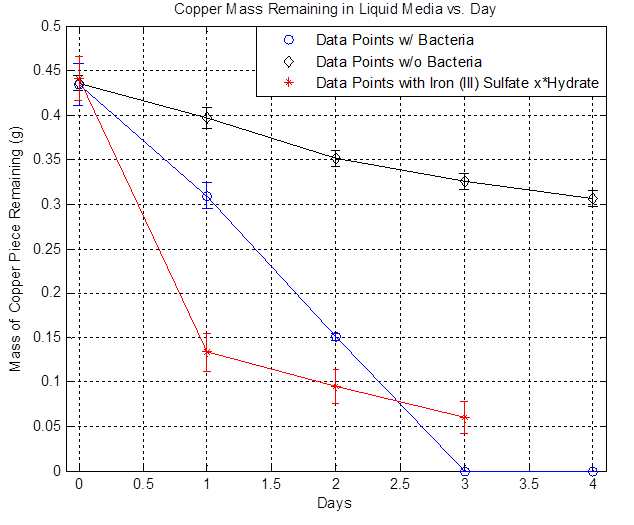
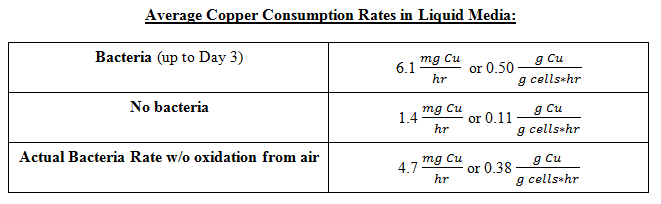
The plot above shows the remaining mass of the copper vs. days. The black line represents the data without bacteria and the decrease in copper mass over time indicates that there is some sort of background etching, which is due to side reactions happening such as the oxidation of copper from air. The blue line represents the data with bacteria and it shows that the copper mass decreased to zero after day 3. The red line represents the data with iron (III) sulfate hydrate and it shows that a significant drop in copper mass after Day 1 compared to the bacteria line because of the immediate availability of iron (III) ions. However, the rate of decrease slows down over time because iron (III) ions are depleted. In the case of bacteria, the iron (II) ions present in the media is converted to iron (III) ions from the bacteria's mechanism, the iron (III) reacts with copper and regenerates iron (II), and iron (II) can be converted to iron (III) once again from the bacteria, resulting in a self-sustaining cycle that eventually depletes all of the copper. Thus, the data shows that the bacteria can accelerate the etching of copper in liquid media and the following copper consumption rates were obtained:
Note that the bacterial rate is ~3x greater than the background rate or that the bacteria increase the etching by ~230% relative to the background rate.
Solid Media
In order to achieve controlled etching, liquid media cannot be used because the bacteria is mobile. Therefore, solid media is explored. (Refer to Solid Media Protocol for solutions used and how to make solid media.) 15 mL of solid media are poured onto a petri dish and four pieces of 2 cm x 2 cm copper are placed inside the media and allowed to harden. Afterwards, ~ 5 mL of OD 15 bacteria (as well as no bacteria) are placed on top of the solid media, spread evenly using sterile glass beads, and placed in an incubator at approximately room temperature for 12 days. Every 3 days, a petri dish with bacteria and a petri dish without bacteria are taken out and all the copper pieces are massed and thrown out.
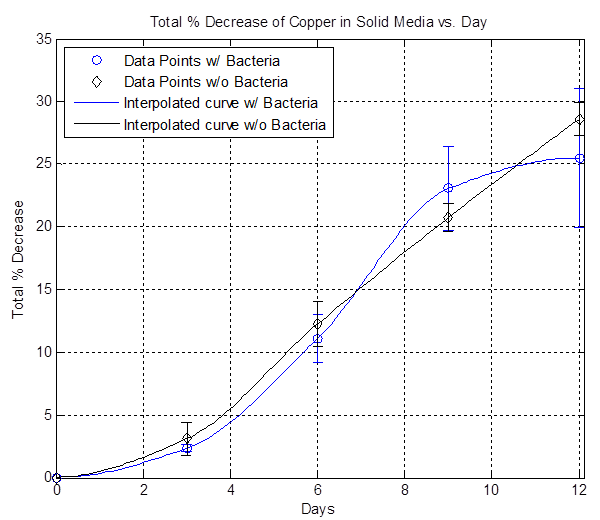
The plot above shows the total average % decrease of copper vs. days. The data here is inconclusive because most of the data points are very close to each other, suggesting that the bacterial etching rate is slow and not faster than the background etching rate.
Hybrid Media
Since bacteria will not accelerate the etching of copper in solid media alone while bacteria in liquid media does, a combination of liquid and solid media (or hybrid media) will be explored.
To make sure that hybrid media can work, experiments were done where cubes of solid media enclosed copper pieces were placed in flasks with 100 mL liquid media containing a pellet of bacteria in a shaker. Controls without bacteria were also used. After shaking for several days, and measuring the masses of the copper, it was found that the flasks with bacteria in them had on average ~11.6% copper mass lost since the start while the flasks without bacteria had on average ~1.4% copper mass lost since the start. This shows that bacteria can accelerate the etching of copper in hybrid media. The problem now is to decrease the amount of liquid media used.
The set-up for the hybrid experiments is the same as the one for solid media except that variable amounts of liquid media are added on top of the solid media before bacteria (if any) were inoculated.
The plot above shows the average % copper lost due solely to the bacteria (bacteria - background) for different amounts of liquid media on solid media. As long as the data points are above zero, the bacteria accelerates the etching of copper in hybrid media using the corresponding amount of liquid media. Thus, volumes of down to 2 mL still work, albeit slow, and we have narrowed the range of liquid media down to between 1 and 2 mL. Note that this doesn't work for the 1 mL case because of the negative percents.
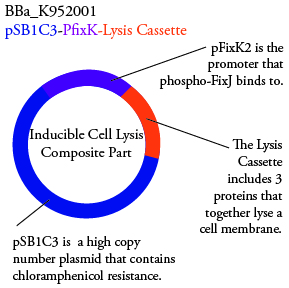
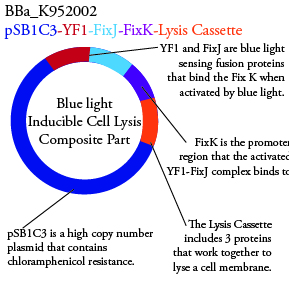
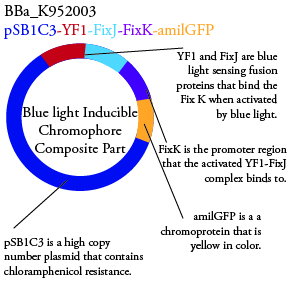
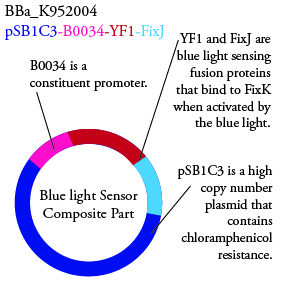
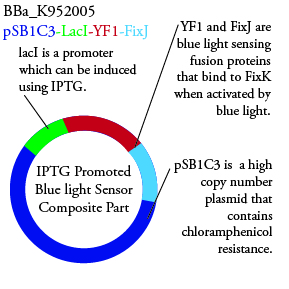
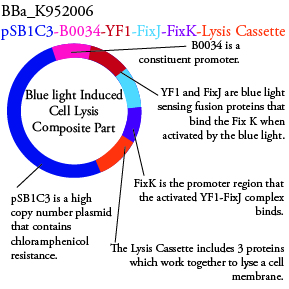
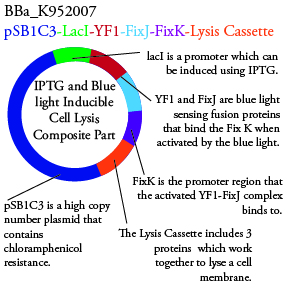
Plasmids Submitted to the Parts Registry:
.
.
.
.
.
.
We plan to characterize parts BBa_K952006 and BBa_K952007 in E. Coli:
For BBa_K95006, we can grow the cells in the absence of IPTG, then add the IPTG and shine blue light onto the culture to induce expression of the lysis cassette killing the cells. We will run 2 controls, the first we will add IPTG but keep the cells in the dark and the second we will shine blue light on the cells in the absence of IPTG. Neither of the controls should cause apoptosis while the culture with both promotors present should.
For BBa_K95007, we do not have the additional IPTG promotor present so we will grow the cells in the absence of light, then shine blue light on it to cause cell death. As controls, we can attempt to grow the cells in the ambient light of the lab, under red light, and then shine blue light on these cultures if they grow. This is to provide information on how sensitive the system is to blue light. Ideally the cells would be able to grow in the ambient light of the lab without inducing cell lysis. However, we do not expect this to be the case.
Depth Etching Rate
As an attempt to obtain a bacterial depth etching rate (the genetically modified A. Ferrooxidans isn't ready yet), experiments using hybrid media (15 mL of solid and of liquid media) with four pieces of 2 cm x 2 cm copper in each petri dish as well as 1 mL OD 0.5 bacteria (if non-control cases) were placed in an incubator. In addition, the coppers were painted with nail polish everywhere but a strip down the center on both sides, which we found out prevents etching from occurring. The nail polish simulates the final result with the genetically modified bacteria because it acts as the illuminated spots that trigger cell death and thus no etching in that region. The masses were obtained after several days and the density of copper along with the non-lacquer covered surface area were used to calculate a depth rate.
The plot above shows the average depth etched vs. days and by subtracting the slopes, the bacteria alone provides a depth etching rate of approximately 2.24 microns/day.
 "
"