Team:Arizona State/Notebook
From 2012.igem.org
(Difference between revisions)
Rohit Rajan (Talk | contribs) (→June 12) |
Rohit Rajan (Talk | contribs) (→June 12) |
||
| Line 35: | Line 35: | ||
** lac o: lots of growth | ** lac o: lots of growth | ||
** neb 10 and dh5a: some growth | ** neb 10 and dh5a: some growth | ||
| - | [[File: ASUiGEM2012_plates061212.jpg|200px] | + | [[File:ASUiGEM2012_plates061212.jpg|200px] |
==June 13== | ==June 13== | ||
Revision as of 06:55, 3 October 2012
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
June 07
- Transformation (LSE)
- Transformed DNA:
- lacZ (well 4:12G, I732019)
- p + lacO (well 1:6G, R0011)
- Cells: neb10beta (donated)
- Protocol from: http://www.neb.com/nebecomm/products/productc3019.asp
- Controls: puc19, no DNA (8 plates)
- Transformed DNA:
June 08
- Transformation results
- puc19: growth
- negative control: no growth
- lacZ, lacO: possible small colonies
- liquid culture in amp media (100 ug / ml):
- no growth of lacZ, lacO
- growth of puc19
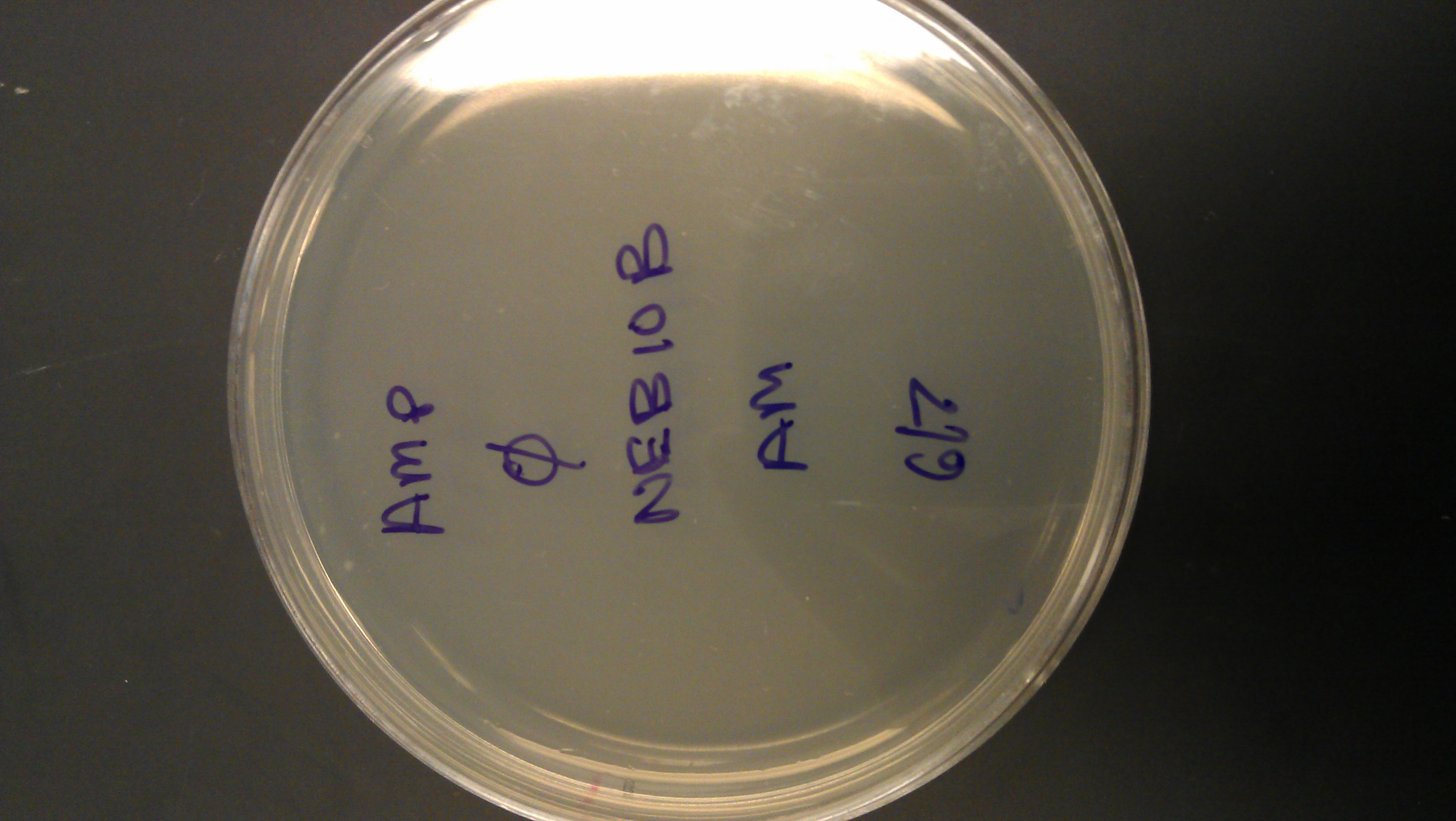
June 12
- DH5a Competent Cell Prep
- Streak plated cells on LB no amp plate, let grow overnight
- Transformation results
- lac z: some growth
- lac o: lots of growth
- neb 10 and dh5a: some growth
[[File:ASUiGEM2012_plates061212.jpg|200px]
June 13
- Transformation (LSE)
- Transformed DNA:
- lacZ (well 4:12G, I732019)
- p + lacO (well 1:6G, R0011)
- Cells: DH5 alpha (donated)
- Protocol from: http://openwetware.org/wiki/Haynes:Assembly101 (30 minute transformation)
- Controls: puc19, no DNA (8 plates)
- Transformed DNA:
- Transformation (istb4, Abhi)
- Transformed DNA:
- Cells:
- Protocol from:
- Controls:
- DH5a Chemically Competent cell prep
- Grew 2 seed colonies from streak plate in LB no amp
- Grew controls to test for contamination
- Both Seed colonies grew, no contamination present
June 14
- Competent cell prep
- Prepared CaCl2 buffer solution and CaCl2 glycerol buffer solution
- Grew seed colony in 400mL LB no amp
June 15
- Competent cell prep
- Centrifuged falcon test tubes containing liquid colonies
- Resuspended in CaCl2 buffer solution and incubated for 15 mins
- Centrifuged and resuspended in CaCl2 glycerol buffer solution
- Chilled overnight
June 16
- Competent cell prep
- Aliquotted 200uL into test tubes
- Stored in -80C
June 17
- Streak plated prepared competent cells on LB no amp plate
- Colonies observed
June 19
- Transformation (LSE)
- Transformed DNA:
- T7 promoter BBa_I712074
- Constitutive promoter BBa_J23102
- Cells: DH5 alpha (donated)
- Protocol from: http://tools.invitrogen.com/content/sfs/manuals/subcloningefficiencydh5alpha_man.pdf (30 minute transformation)
- Controls: puc19, no DNA
- Plated 2 copies of each (100 ul, 250 ul) on LB amp plates.
- Transformed DNA:
- Made 50 LB Amp plates.
June 20
- Plated negative control on LB Amp plate
- Liquid cultures of T7 promoter and constitutive promoter
- Transformation (LSE)
- Transformed DNA:
- RBS (well 1:1H BBa_B0030)
- TetR GFP (well 2:8A Part:BBa_I13522)
- Cells: DH5 alpha (donated)
- Protocol from: http://tools.invitrogen.com/content/sfs/manuals/subcloningefficiencydh5alpha_man.pdf (30 minute transformation)
- Controls: puc19, no DNA
- Transformed DNA:
June 21
- Made Liquid Cultures of E.coli transformed with RBS B0030
- Made Liquid Cultures of E.coli transformed with TetR GFP
- miniprepped and nanodropped T7 promoter BBa_I712074 and Constitutive promoter BBa_J23102 liquid cultures
- liquid cultures:
- RBS1
- RBS2 (duplicate_
- GFP1
- puc19
- negative controls
- 5 ml LB amp
- overnight cultures
- replated GFP1 & 2 (duplicates)
- Nanodropped plasmid DNA samples
- Constitutive promoter 1: 2.554 ng/uL
- Constitutive promoter 2: 2.345 ng/uL
- T7 promoter 1: 3.369 ng/uL
- T7 promoter 2: 3.049 ng/uL
June 22
- Miniprepped liquid cultures: RBS (well 1:1H BBa_B0030) and TetR GFP (well 2:8A Part:BBa_I13522)
- Picked colonies:
- 1 colony from double terminator (dt1) plate
- 1 colony from t7 polymerase (pol1) plate
- 1 colony from puc19 plate (positive control)
- 1 colony from dh5a plate (negative control)
- started liquid cultures of each colony (5 mL LB amp each)
June 26
- Transformation:
- Transformed DNA:
- double terminator (B0017, 2:6K)
- T7 RNA polymerase (I715038, 2:15C)
- puc19, negative control
- Protocol from: http://tools.invitrogen.com/content/sfs/manuals/subcloningefficiencydh5alpha_man.pdf (30 minute transformation)
- Cells: dh5a
- Transformed DNA:
June 27
- 6-26 transformation results:
- Controls correct
- 2x terminator: ~19 colonies
- RNA pol: 1 colony
- Liquid cultures including controls
June 28
- Miniprepped double terminator (B0017, 2:6K) and T7 RNA polymerase (I715038, 2:15C) liquid cultures
July 2
- Cleaned up liquid waste
- Made SOB media
- Finalized oligos for magainin construct
July 3
- Autoclaved SOB media
- Added glucose to make SOC media
- Nanodropped double terminator (B0017, 2:6K) [DT1: 24.5, DT2: 29.6] and T7 RNA polymerase (I715038, 2:15C) [P1: 64.6, P2: 55.3] liquid cultures
July 15
- Ran a miniprep of Bgal alpha 1&2, T7 and PSV
- Procedure from GenElute HP PLasmid Miniprep Kit (Sigma-Aldrich)
July 16
- Transformation of PLflex (part: BBa_J176040) and PLflex4 (part: BBa_J176130) linkers.
- Incubate at 37C for 8 hours for DH 5alpha turbo cells
- Inoculated 5 mL LB/AMP media in 15 mL culture tubes and added the colony. Incubated in 37C overnight.
July 17
- Miniprep of pLFlex DH5alpha and pLFlex 4 DH5alpha
- Procedure comes from Zymo Research's Zyppy Kit.
- Ran the spectrophotometer to determine the concentration for the miniprep DNA for the linker pLFlex and linker pLFlex 4
- Concentrations were:
- pLFlex: 73.792 ng/uL
- pLFlex 4: 38.587 ng/uL
- Absorbance ratios were:
- pLFlex: 1.864
- pLFlex 4: 1.851
- Ran the miniprep for pLFlex and pLFlex 4 again.
- Same procedure
- Ran the spectrophotometer again
- Concentrations were:
- pLFlex: 48.095 ng/uL
- pLFlex 4: 18.214 ng/uL
- Absorbance ratios were:
- pLFlex: 1.79
- pLFlex 4: 1.85
- Transformation was successful but 8-9 hour growth period was not sufficient for DH5 alpha.
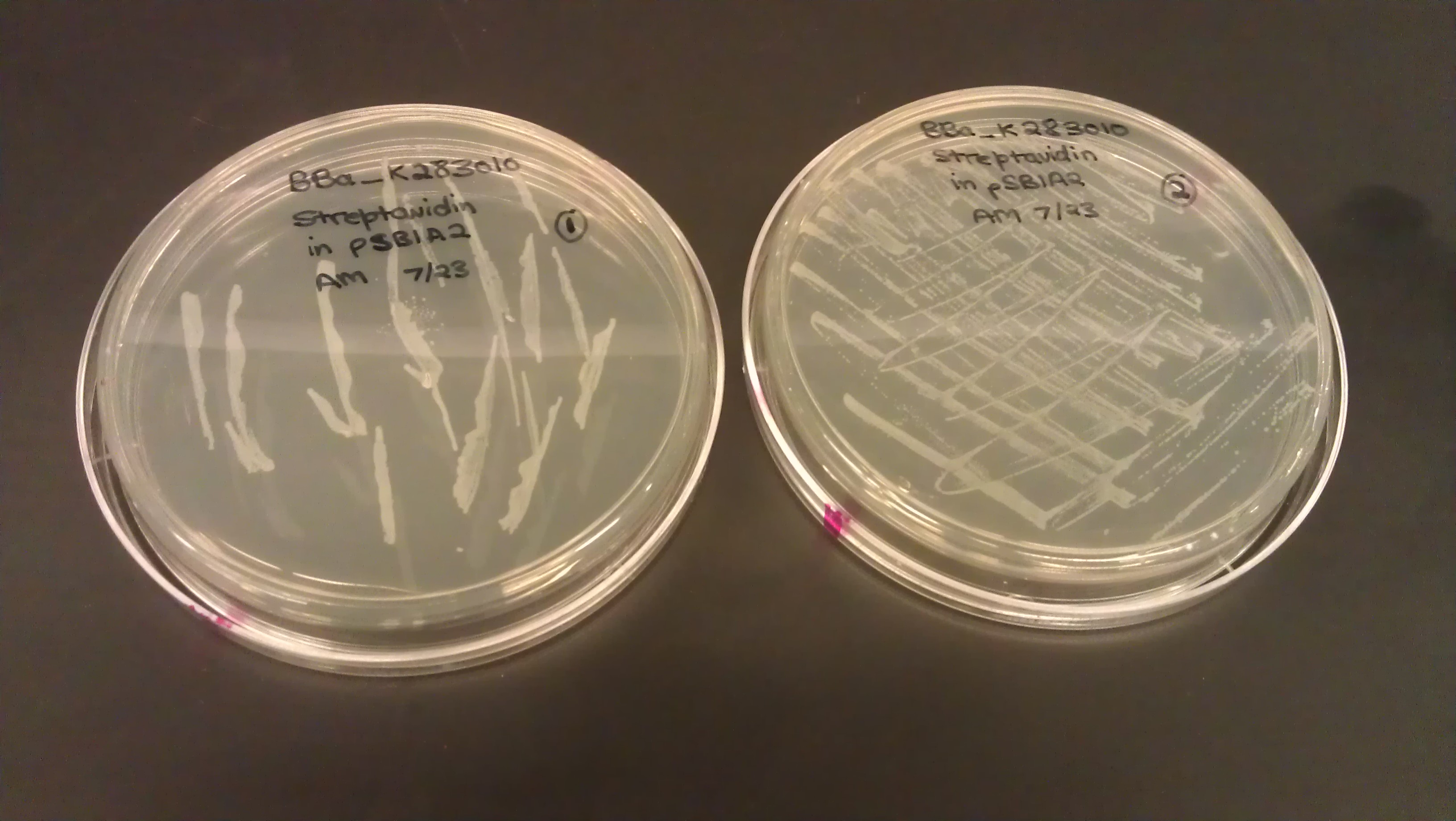
July 23
- Plated BBa_K283010 (Streptavidin) on LB amp plate from the agar stab provided from the iGEM head-quarters.
- Incubated the dish overnight at 37 C.
- Plate 1: pipet tip, colonies are in wells, neither distinct nor segregated
- Plate 2: inoculating loop, numerous non-distinct colonies on the plate
July 24
- Plasmids arrived courtesy of University of Pennsylvania School of Medicine
- pET29a vectors containing coding sequence for Topoisomerase mutants CSCS and CSCS D168A described here
July 25
- Ran a miniprep of streptavidin BBa_K283010
- Protocol from Zymo Research's Zyppy Plasmid Miniprep Kit
- Tranformed CSCS topo 0 plasmid and CSCS D168A topo into DH5a Turbo cells (with neg control and Puc19 neg control)
- Plated on Kanamycin plates
July 26
- Picked colonies colonies from Topo 0 and Topo D168A and grew liquid cultures in Kan media
July 27
- Miniprepped liquid colonies and nanodropped.
- Plasmid concentrations
- Topo O:
- Topo D168A1:
- Topo D168A2:
July 30
- Prepared Kan Media and Kan Plates
July 31
- PCR amplified polylinker sequence of Topo plasmid with Promega GoTaq protocol
- Used pET29a upstream forward primer and T7 terminator reverse primer
August 3
- Submitted pET29a Topoisomerase plasmid to Biodesign for sequencing
- Resuspended GFPT1 and GFPT2 oligos with molecular grade (nuclease-free) H2O.
- Final Concentration 100uM
- (gfpt1 top1, gfpt2 top1, gfpt1 top2, gftp2 top2, gfpt1 bot1, gfpt2 bot1, gfpt1 bot2, gfpt2 bot2)
- (3uL of each oligo + 2uL 10x annealing buffer, 6uL molecular grade H2O. 20uL Reactions)
- Final Concentration 100uM
- Heated for 5 minutes at 100C. Let cool to room temperature on the heating block, stored at -20C.
- Digested BBa_I13522 with XbaI and PstI.
- Attempted ligating annealed oligos into a digested plasmid from Ryan (realized it was cut with E and P).
August 6
- Annealed oligos for GFPT1 and GFPT2 (target probes)
- Ligated oligos with digested GFP plasmid (BBa_I13522)
- Transformed into competent DH5alpha
- Added SOC and incubated at 37C for 15 minutes.
- Plated on amp treated plates.
August 7
- Only one colony on each plate (both were white)
- Picked colonies and started 5mL LB amp cultures of each, stored at 37C
- Stored plates in 37C
August 8
- Picked the colonies again and started new liquid cultures (5mL LB amp).
- Discarded cultures for 8/7/12
August 9
- Miniprepped 3mL of each 8/8/12 culture and nanodropped:
- gfpt1 - 155 ng/uL
- gfpt2 - 114 ng/uL
- Digested gfpt1 and gfpt2 with X and P
- Ran on a 1% agarose gel with the digested GFP plasmid
- Made glyercol stocks with aliquot of the remaining liquid cultures
August 10
- PCR of Alpha-4, 1-omega, omega, and alpha fragments using corrected primers
August 13
- Ran gel of split beta gal fragments. Confirmed 3 out of the 4 fragments except for the alpha-4 fragment.
- Prepared sequencing samples
- Sample w/ Primer:
- GFPT1 w/ VF2 GFPT1 w/ VR GFPT2 w/ VF2 GFPT2 w/ VR
- 200ng of DNA + 16 pmol of primer
- Annealed oligos again GFPT1/2
- Repeated ligation of oligos with digested GFP plasmid (BBa_I13522)
- Followed Haynes assembly protocol instead of standard DH5alpha protocol. (http://openwetware.org/wiki/Haynes:Assembly101)
- Transformed ligations into competent DH5alpha
- Plated on amp treated plates
August 14
- Took pictures of plates
- Green-white screened plates
- Picked 4 white colonies from each of gfpt1/2 plates
- Made 5mL LB amp cultures of each colony
- Delivered GFPT1/2 dna samples to biodesign for sequencing (samples from 8/13/12)
- Assembled magainin insert via Overlapping oligo assembly
- Digested pUC 19 plasmid with EcoRI and PstI
- Transformed Magainin insert into digested pUC 19 plasmid. Failed. Probably too much X-gal on plate.
Ran gel for the beta gal alpha-4 fragment. Failed. Fragment not in the correct size-band.
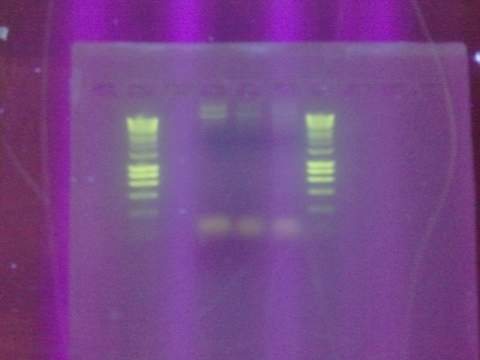
August 15
- [http://129.219.2.10/home2/dnalims/fragment/32/21/pfasta/topo0_T7Term.seq Topo 0], [http://129.219.2.10/home2/dnalims/fragment/32/21/pfasta/D168Atopo1_T7Term.seq D168A Topo1], and [http://129.219.2.10/home2/dnalims/fragment/32/21/pfasta/D168Atopo2_T7Term.seq D168A Topo2] sequence results
- Miniprepped 3mL of each liquid culture of GFPT1/2
- Prepared glycerol stocks using 100uL of each liquid culture
- Nanodropped samples:
- GFPT1-1 - 172.6 ng/uL
- GFPT1-2 - 203.7 ng/uL
- GFPT1-3 - 197.4 ng/uL
- GFPT1-4 - 178.9 ng/uL
- GFPT2-1 - 107.3 ng/uL
- GFPT2-2 - 131.2 ng/uL
- GFPT2-3 - 145.5 ng/uL
- GFPT2-4 - 172.0 ng/uL
August 16
- Replated magainin insert + plasmid into the grid. Failed. All blue colonies meaning that no insert.
Tried gel for all gel-isolated fragments. Failed. Did not get a band in the 2000 bp region. Only got things below 200 bp.
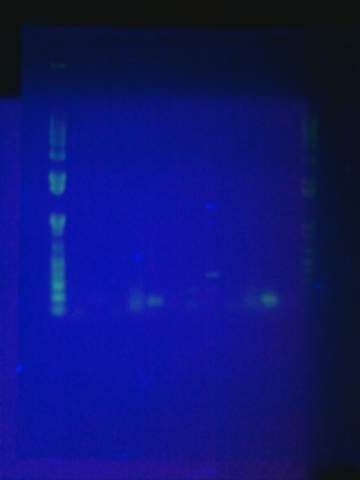
August 17
- GFPT1 sequence confirmed
- Prepared aliquots of GFPT2 minipreps from 8/15/12 for sequencing
- Delivered GFPT2 samples to biodesign for sequencing
August 19
- Restricted PLFlex and PLFlex 4 with ecori and xbai.
- Restricted Bgala with Xbai and psti
- Used Hayne's Lab Protocol
- GFPT2 sequences confirmed
August 27
- Revived GFPT1 (from 8/9/12) and GFPT2 (2-2 from 8/15/12) cultures from glycerol scrapes
- Made 4mL cultures in LB Amp
August 29
- Discarded GFPT1/2 cultures from 8/27/12
- Revived GFPT1 (from 8/9/12) and GFPT2 (2-2 from 8/15/12) cultures from glycerol scrapes
- Made 4mL cultures in LB Amp
- Digested GFPT1/2 with X and S
- Ran a 1% agarose gel with GFPT1/2 digestions
- Cut out inserts and GFPT2 backbone and stored in 4C for gel extraction and tandum repeat assembly experiments
August 30
- Prepared extra glyercol stocks of GFPT1/2 cultures from 8/29/12
- Miniprepped 3mL of each culture, stored at -20C
September 19
- Set up VF2/VR endpoint PCR for double transform minipreps
- 1-1, 1-1I, 1-2, 1-2I, 1-3, 1-3I, 2-1, 2-1I, 2-2, 2-2I, 2-3, 2-3I, GFPT1 (positive controls), GFPT2 (positive controls)
- Annealing temp set to 55C for 25 cycles
- Resuspended GFPT1 probe and GFPT2 probe oligos in molecular grade H2O (Final concentration: 100uM), stored at -20C
September 25
- Resuspended pSB1A2 FWD and pSB1A2 REV (amp resistance primers) oligos in molecular grade H2O (Final concentration: 100uM), stored at -20C
- Prepared 1.6uM dilutions (500uL)
- Did endpoint PCR using pSB1A2 primer pair on
- Topo, Topo IPTG, Topo D168A, Topo D168A IPTG, 1-1, 1-1I, 2-1, 2-1I, GFPT1, GFPT2
- Did endpoint PCR using VF2/VR primer pair on
- Topo, Topo IPTG, Topo D168A, Topo D168A IPTG
- Annealing temp 55C for 25 cycles, stored products at -20C
- Made 3mL liquid cultures of the shipping vector (pSB1C3 with RFP insert) in DH5alpha in chloramphenicol resistant LB
- Made 10mL liquid cultures of:
- Topo in kanamycin
- topo D168A in kanamycin
- topo + GFPT1 in kanamycin + ampicillin
- topo + GFPT2 in kanamycin + ampicillin
- stored @ 37C
September 26
- Picked two new colonies for topo + GFPT2 and grew 10mL cultures of amp+kan LB broth for each
- Ran a gel with:
- Hyperladder I, GFPT1 pSB1A2, GFPT2 pSB1A2, Topo pSB1A2, Topo D168A
- Ran a gel with PCR samples from 9/19 and 9/25:
- Hyperladder I, 1-1, 1-1I, 2-1, 2-1I, 2-1I(VF), 2-1(VF), 1-1I(VF), 1-1(VF), Hyperladder I
- Samples in wells 2-5 used the pSB1A2 primer pair. Samples in wells 6-9 used VF2/VR primer pair.
- Revived GFPT1/2 from glycerol stocks (from 8/9 and 8/15) in 5mL LB amp each.
- Nanodropped miniprepped DNA samples:
- pSB1C3 I - 253.75 ng/uL
- pSB1C3 II - 258.38 ng/uL
- Topo - 24.84 ng/uL
- Topo I - 17.33 ng/uL
- Topo D168A - 7.24 ng/uL
- Topo D168A I - 15.62 ng/uL
- 1-1 - 16.49 ng/uL
- 1-1I - 16.46 ng/uL
- 2-1 - 142.05 ng/uL
- 2-1I - 16.98 ng/uL
- Picked new colonies from Topo, Topo D168A, Topo + G1, Topo + G2 and made 1mL colonies in their respective medias
- Stored at -37C
September 27
- Prepared serial dilutions of GFPT2 plasmid 1:10, 1:100, 1:1000, 1:10000
- Prepared primer mixes for pSB and VF/VR primers
- Prepared realtime PCR plate, ran RT-qPCR with annealing temp 57
- Miniprepped GFPT1/2 cultures from 9/26
- Nanodropped Miniprepped DNA:
- GFPT1 - 276.12 ng/uL
- GFPT2 - 219.84 ng/uL
- Used 100uL of each 1mL culture from 9/26 to seed 10 mL cultures in their respective media
- Added 10uL of 1M IPTG to each culture ~4 hours after seeding
- Removed cells from 37C ~4 hours after IPTG inducing
- Pelleted and lysed following the bugbuster protocol (http://openwetware.org/wiki/User:Behzad_Damadzadeh/Notebook/PcTF_Subcloning_in_E-coli/2012/05/22) (used lysonase for Topo and Topo D168A only)
- HIS purified proteins using the Zymo HIS purification kit
- Digested pSB1C3 plasmid with X and P
- Sent protein samples (HIS purified samples from 9/19, 20uL) and ssDNA control (GFPT1/2 probe oligos 20uL @ 10uM) for mass spec
September 28
- Resuspended 8 new oligos, final volume 100 uM each
- Annealed pet29 top/bot oligos
- Redid the RT-PCR, 3x primer concentration, added 1:1 plasmid concentration
- Made 1.6uM aliquots of primer stocks 1-7 (including topo add X primer previously ordered)
- Diluted aliquot of Topo D168A plasmid 4:10
- Diluted PSV plasmid 2:20
- Performed Endpoint PCR on:
- Topo D168A using primers 1,2 and primers 1,3
- PSV using primers 4-5 and primers 6-7
- 4 samples with low primer concentration, 4 samples with twice as much primer (labelled 'H')
- Miniprepped Topo1 2, Topo1 3, Topo1 4 (biobricked Topo D168A without T7, multiple colonies from ligation into shipping vector)
- Nanodropped:
- Topo1 2 - 63.63 ng/uL
- Topo1 3 - 75.07 ng/uL
- Topo1 4 - 184.93 ng/uL
- 1mL chloramphenicol cultures of GFPT1/GFPT2 in shipping vector prepared
- Digested Topo1 2, Topo1 3, Topo1 4 with E and P
- Ran digested Topo plasmids on 1% agarose gel with Hyperladder I
- Revived cultures of 2xGFPT1, 2xGFPT2, Topo, and Topo D168A (4 mL cultures each in their respective medias)
- T5 exonuclease treated miniprepped plasmids (1-1, 1-1I)
- A1 1-1I + t5
- B1 1-1 + t5
- A2 1-1I untreated
- B2 1-1 untreated
September 29
- Miniprepped GFPT1, GFPT2, Topo, and Topo D168A (Topo cultures separated into 3mL and 1mL minipreps)
- Nanodropped miniprepped DNA:
- GFPT1 I - 275.29 ng/uL
- GFPT1 II - 101.08 ng/uL
- GFPT2 I - 222.11 ng/uL
- GFPT2 II - 230.52 ng/uL
- Topo I - 117.97 ng/uL
- Topo II - 70.28 ng/uL
- Topo D168A I - 69.47 ng/uL
- Topo D168A II - 51.87 ng/uL
- HIS purified crude lysates from 9/27/12 (Topo, Topo D168A, Topo + GFPT1, Topo + GFPT2, all IPTG induced)
- Digested Topo I plasmid (pet29a) with E and X
- Ran a gel with Hyperladder I, 1-2 1-3 4-5 and 6-7 PCR products, pet29a digestion, and pSB1C3 digestion
- Confirmed that PCR made amplicons
- Excised bands for digested pet29a and pSB1C3 plasmids
- Gel extracted 4 gel fragments (2 wells per sample: digested pSB1C3 plasmid, digested pet29a plasmid)
- Nanodropped gel extractions:
- Digested pSB1C3 - 25.54 ng/uL
- Digested pet29a - 22.95 ng/uL
- Set up a bradford assay of topo, topo D168A, topo + G1, topo + G2 (5uL protein, 10uL protein, 20uL protein + 200uL reagent)
- Used ~100uL aliquot of BL21 competent glycerol stock to seed 10mL of LB medium (no antibiotic), stored at 37C
- Ran 1% agarose gel with samples: A1, B1, A2, B2 and Hyperladder I
- Did bug buster protocol to lyse BL21 control culture (used lysonase)
- Ligated digested pet29a with pet29 top/bot annealed oligos
- Transformed ligation using invitrogen DH5alpha transformation protocol
- Prepared LB kanamycin plates
- plated transformed ligations on prewarmed kanamycin plates
September 30
- Pet29a plates did not grow
- Kinase treated pet29a oligos
- Annealed kinase treated pet29a oligos
- Ligated digested pet29a (from gel extraction) with kinase treated oligos
- Transformed ligations into DH5alpha, used topo plasmid as a positive control
- Plated transformations on kanamycin plates and stored overnight at 37C
- HIS purified BL21 control crude lysate
- Set up a bradford assay with:
- uninduced & induced topo protein extractions from 9/19
- uninduced & induced topo D168A protein extractions from 9/19
- BL21 control lysate
- Topo, Topo D168A, Topo + G1, Topo + G2 from 9/27
- All samples prepared (10uL protein, 20uL protein + 200uL reagent)
- Miniprepped GFPT1 1,2,3 and GFPT2 (17,18,26) (~600uL of each) (1mL liquid cultures made from colonies on the chloramphenicol plates of GFPT1 and GFPT2 ligated into the shipping vector)
- Nanodropped:
- GFPT1-1 - 38.5 ng/uL
- GFPT1-2 - 77.6 ng/uL
- GFPT1-3 - 74.8 ng/uL
- GFPT2-17 - 61.6 ng/uL
- GFPT2-18 - 65.9 ng/uL
- GFPT2-26 - 64.2 ng/uL
- Ran a 1% agarose gel with 1-1, 1-2, 1-3, 2-17, 2-18, 2-26 plasmid miniprep samples and Hyperladder I
- Prepared a 1:2 dilution of GFPT2 plasmid from 9/27 miniprep
- Treated 5uL of diluted plasmid with 5uL of water, BL21 protein, topo protein, topo D168A protein
- Incubated 30 minutes at 37C
- Ran a 1% agarose gel with protein treated target plasmid samples and Hyperladder I
- Digested GFPT2 plasmid with X (let run at 37C for 30 minutes)
- Used DNA clean up kit on digested GFPT2
- Nanodropped digested GFPT2:
- GFPT2(X) - 39.85 ng/uL
- Prepared DNA seq samples using VF2 and VR
- Sample# - PrimerPair - DNA sample (sample 1, GFPT2 uncut + VF2; sample 2, GFPT2 uncut + VR)
- 1/2 - FWD/REV - uncut GFPT2 plasmid
- 3/4 - FWD/REV - cut GFPT2 plamid
- 5/6 - FWD/REV - 2:1 uncut:cut GFPT2 plasmid mixture
- 7/8 - FWD/REV - 1:1 uncut:cut GFPT2 plasmid mixture
- 9/10 - FWD/REV - 1:2 uncut:cut GFPT2 plasmid mixture
- 11/12 - FWD/REV - 1-2 miniprep sample from 9/19 double transformations
- 13/14 - FWD/REV - 1-2I
- 15/16 - FWD/REV - 1-3
- 17/18 - FWD/REV - 1-3I
- 19/20 - FWD/REV - 2-1
- 21/22 - FWD/REV - 2-1I
- 23/24 - FWD/REV - 2-2
- 25/26 - FWD/REV - 2-2I
- 27/28 - FWD/REV - 2-3
- 29/30 - FWD/REV - 2-3I
October 1
- Minipreps of:
- J61011 + S + L + ALPHA 2B
- J61100 + S + L + ALPHA 2B
- J61100 + S + L + ALPHA 2C
- PLUX2 + RBS + ALPHA 1A
- PLUX2 + RBS + ALPHA 1A (2)
- PLUX + S + L + ALPHA 2C
- J61101 + S + L + ALPHA 1A
- J61101 + S + L + ALPHA 1A (2)
- PLUX2 + RBS + S + L + ALPHA 2B
- PLUX2 + RBS + S + L + ALPHA 2B (2)
- J61101 + S + L + ALPHA 2C
- PLUX + S + L + ALPHA 2B
- Ran Twice when finding concentrations:
- J61101 + S + L + ALPHA 1A
- J61101 + S + L + ALPHA 2B
- Restricted all the above and:
- Strep + Linker + OMEGA
- Strep + Linker-4 + OMEGA
- with:
- X+P
- Ligated into pSB1C3 shipping vector and transformed into BL21(DE3) cells.
- Prepared all 14 above samples for sequencing.
October 2
- Prepared PCR tubes with 10uL Topo1 4 (25ng/uL topo d168a in the shipping vector), 10uL GFPT2-26 (25ng/uL GFPT2 in the shipping vector), and 10uL GFPT1-3 (25ng/uL GFPT1 in the shipping vector)
- Labelled the tubes K891234, K891999, K891000 respectively and shipped overnight to iGEM Headquarters
 "
"