Contents |
Why?
From our work on LOV2 photoactivation we should be able to predict the percentage of LovTAP-VP16 proteins that are photoactivated depending on the irradiance applied.
- Can we then predict the occupancy of TrpO binding sites in our reporter plasmid by LovTAP-VP16 dimers?
- And the difference in expression levels between an irradiated cell culture a one in the dark?
What?
Well, things start to get complicated, since the number of unknowns is huge in this problem, because it's affected by the transient transfection process, the cell's metabolism, the interaction of plasmids with the genomic DNA, the interaction of VP16 with the transcriptional cofactors and lots more.
We can split this into two parts:
- Estimation of the proportion of active plasmids that will be bound to LovTAP-VP16. We will assume the same binding affinity ratio (light/dark) as Strickland et al (2008) found for LovTAP at 25ºC because, although the absolute affinity is temperature dependent, according to Jin et al (1993), this change is not important. We are not sure whether only one or two LovTAP-VP16 dimer can bind a TrpO sequence. According to Yang et al (1996) a whole TrpO sequence can be bound by a tandem of two TrpR dimers. We will modelize both cases.
- Estimation of the expression level of reporter gene, dsRed in this case, depending on the proportion of bound reporter plasmids, based on the estimation from the previous part. Warning! This might give us an idea of the orders of magnitude, but can't be validated until lots of experiments have been done.
How?
Photoactivation level
We will then assume that we know the proportion of activated LovTAP-VP16 monomers, since we have calculated it in this section. But LovTAP-VP16 has to dimerize to be able to bind DNA. To illustrate the importance of this, let's give an example: imagine a solution with LovTAP-VP16 where 50% of the monomers are activated. Assuming that all of them form part of a dimer, we get that 25% of the dimers are inactive, 25% active and 50% half active. The average binding affinity will mostly depend on the affinity of the half active half inactive dimers. In the section on the 3D protein structure of LovTAP-VP16 we conclude that it is reasonable to think that a half activated dimer will have a DNA activity somewhere between a fully activated dimer and a deactivated one. This means that we can take the monomer activation level to approximate the dimer activation level.
Number of bound reporter plasmids
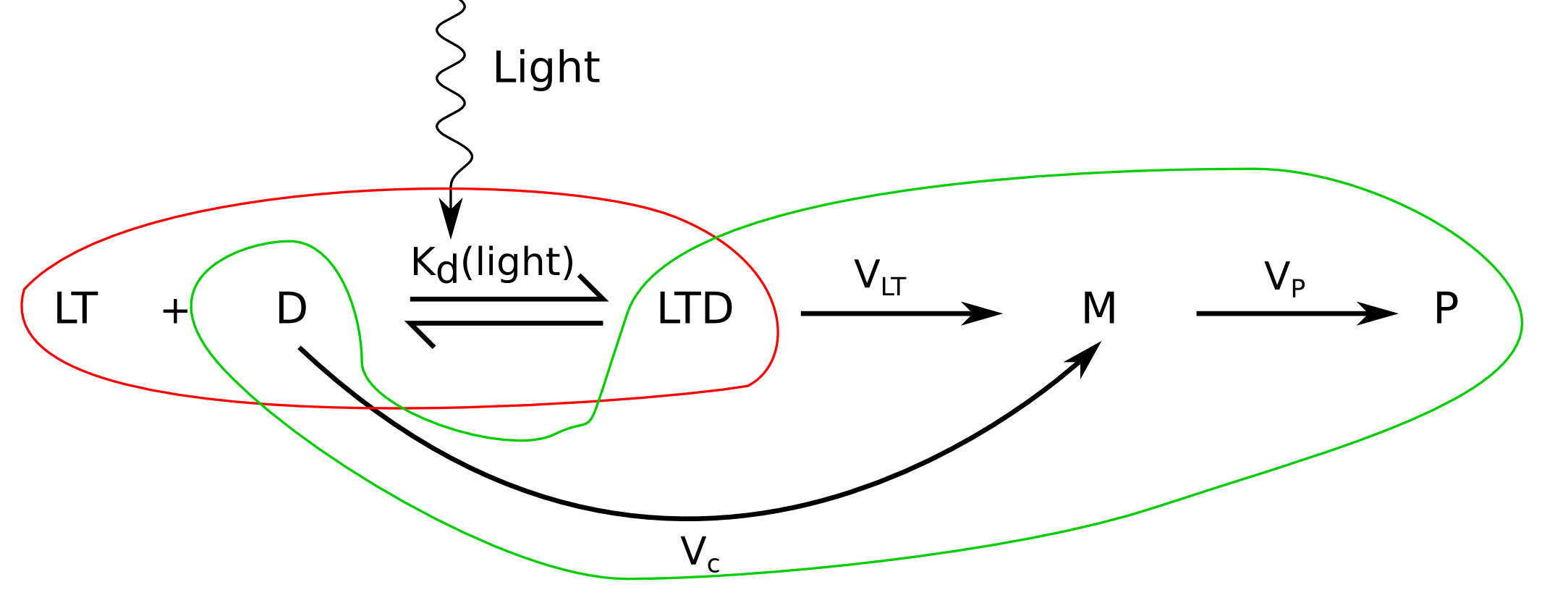
To modelise the behavior of LovTAP-VP16, we will start with the following assuptions:
- [LovTAP-VP16] >> [Plasmids] in the nucleus.
- LovTAP-VP16 is always a dimer and the number of activated LOV2 domains is equivalent to the number of active dimers.
Let's go step by step; if we assume that only one LovTAP-VP16 dimer can bind the TrpO binding site, reporter plasmids can exist in two states; free or bound to a LovTAP-VP16 dimer, and the total number of available plasmids must be the sum of the two:
So far, we have been transfecting our CHO cells transiently using [http://en.wikipedia.org/wiki/Polyethylenimine PEI]. According to our protocol, we add 1.5 µg of DNA per million cells; that is 1.5x10⁻⁶ µg per cell. Our plasmids are about 6000 bp, or around 10⁻¹¹ µg per plasmid. This means we dose some 1.5x10⁵ plasmids per cell. How many can we expect, in average, to reach the nucleus? Well, experience in the lab shows that only some 15% to 30% of the cells show expression. According to Cohen et al, 2009, we could expect somewhere between 100 and 1000 plasmids to reach the nucleus in average. We don't know how many of those will be transcribed, and we don't know how the conditions in the nucleus will affect the way LovTAP-VP16 binds DNA.
From now on we can't expect producing more than qualitative information.
92% of photoactive LOV2 has it's Jα-helix undocked (Yao et al, 2008).
Eitoku et al (2005) say it takes 2 ms for the J&alpha-helix to undock.
 "
"