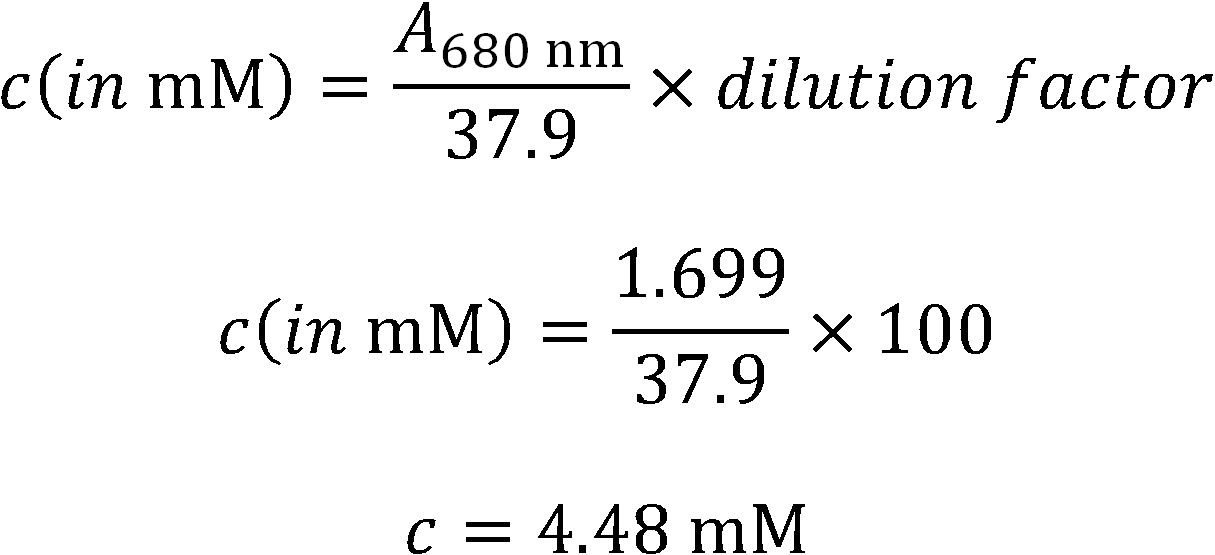Team:TU Munich/Project/Light Switchable Promoter
From 2012.igem.org



Contents |
Light-Switchable Promoter
The so-called "Reinheitsgebot" or "Bavarian Beer Purity Law" forbids the use of any ingredients other than water, barley and hops. Hence, to be able to control the expression of our pathways in yeast, a promoter which does not rely on any chemical additive.
The light switchable promoter, does not only comply with these needs, it is also easy, cheap and very precisely applicable. Furthermore, as the expression of the downstream gene can be up-regulated as well as down-regulated by variation of red light and far red light ratio respectively.
Therefore it allows high spatio-temporal control over the genes downstream of the promoter.
Background and Principles
This system bases on the yeast two-hybrid system which was originally created for exploring protein-protein interactions. One candidate of a potential protein-interaction pair is fused to the DNA-binding domain of a transcription factor and the other candidate to the activation domain of a transcription factor. If the proteins candidates are really physically interacting with each other, this event will starts the transcription of downstream reporter genes, e. g. LacZ or an auxotrophic marker.
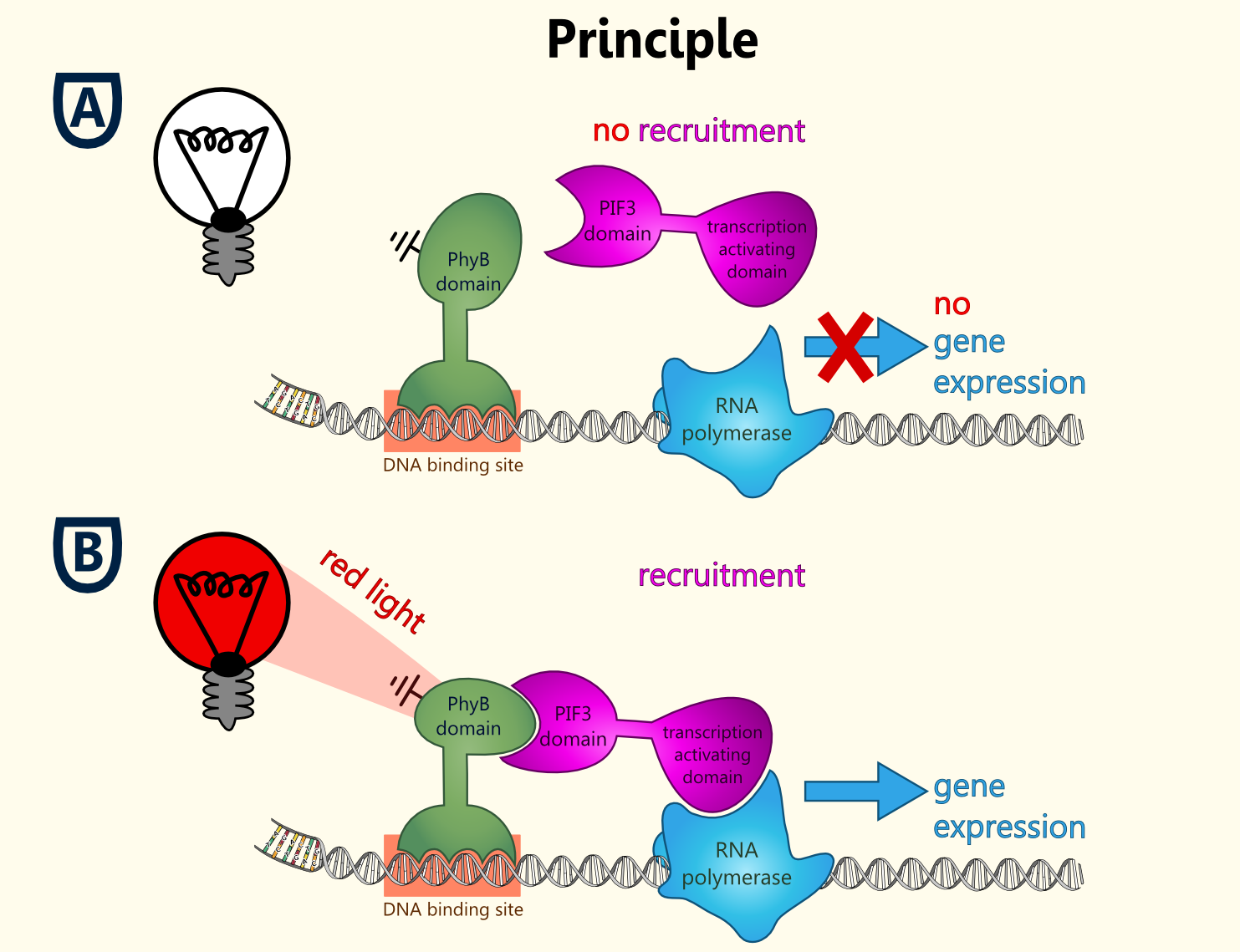
Reverse Yeast-Two Hybrid Based Light-Switchable Promoter System
This basic principle is utilized in the yeast light-switchable promoter system. But in contrast to yeast-two hybrid, we already know the interaction partners (PhyB and PIF3). The photo-convertible binding of PhyB to PIF3 is used, to recover the physical contiguity of the DNA binding domain and the transcriptional activation domain under defined conditions (red light).
This light-inducible system contains two proteins, phytochrome B (PhyB) and phytochrome interacting factor 3 (PIF3). PhyB and PIF3 will just form a heterodimer, if PhyB is exposed to red light. Exposition under red light leads to a conformation change of PhyB to its active form (Pfr-form); the Pfr form of PhyB now can bind PIF3. PhyB comprises a light-absorbing chromophore phycocyanobilin, which gives PhyB the ability to undergo a photoconversion to the active Pfr form (red light exposition) or back to its ground-state Pr (far-red light exposition or darkness).
GAL4 Based Light-Switchable Promoter System
In our first case we create two constitutively expressed fusion proteins, the first one is PhyB fused to GAL4DBD for the DNA binding part ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K801040 BBa_K801040] and the second one is PIF3 fused to GAL4AD for the transcriptional activating part ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K801039 BBa_K801039]). This system allows us to control spatio-temporally the expression of our genes coded on [http://partsregistry.org/wiki/index.php?title=Part:BBa_K801004 pTUM104] and driven by the GAL1 promoter (The TATA-box of pGAL1 is preceded by binding elements for GAL4). To prevent interference with the endogenous GAL4 system of yeast, we are using the Y190 S. cerevisiae strain, which has an GAL4/GAL80 deletion.
One great advantage of the GAL4 based system is that we can use all our constructs which we have first cloned downstream of a GAL1 promoter without further cloning steps! But the disadvantage is that we have to use a yeast strain carrying a GAL4/GAL80 deletion.
If you want to use a supermarket yeast or a brewing strain you have to use the LexA based light-switchable promoter system, described in the next section.
LexA Based Light-Switchable-Promoter System
In contrast to the GAL4 based light-switchable promoter system there is no need for KO of GAL4/GAL80 genes in yeast with a LexA based light-switchable promoter system. The difference is that we use LexA, a prokaryotic DNA binding protein, for the DNA binding part of our light-switchable promoter system, instead of GAL4DBD. LexA does not interfere with the endogenous yeast metabolism and signaling system because it only recognizes a special prokaryotic DNA sequence, the so-called LexA operator (=LexA binding site). LexA binding sites can be used upstream of a minimal promoter (=TATA box) to be utilized as a cis-acting regulatory element.
In this case the genes, which we want to control by light, have to be cloned downstream of a synthetic promoter containing a minimal promoter, preceded by multiple LexA binding sites, e. g. [http://partsregistry.org/wiki/index.php?title=Part:BBa_K165031 BBa_K165031].
In distinction from the GAL4 based system there is no necessity for a special strain carrying an GAL4/80 deletion, so theoretically every yeast strain can be used for this system.
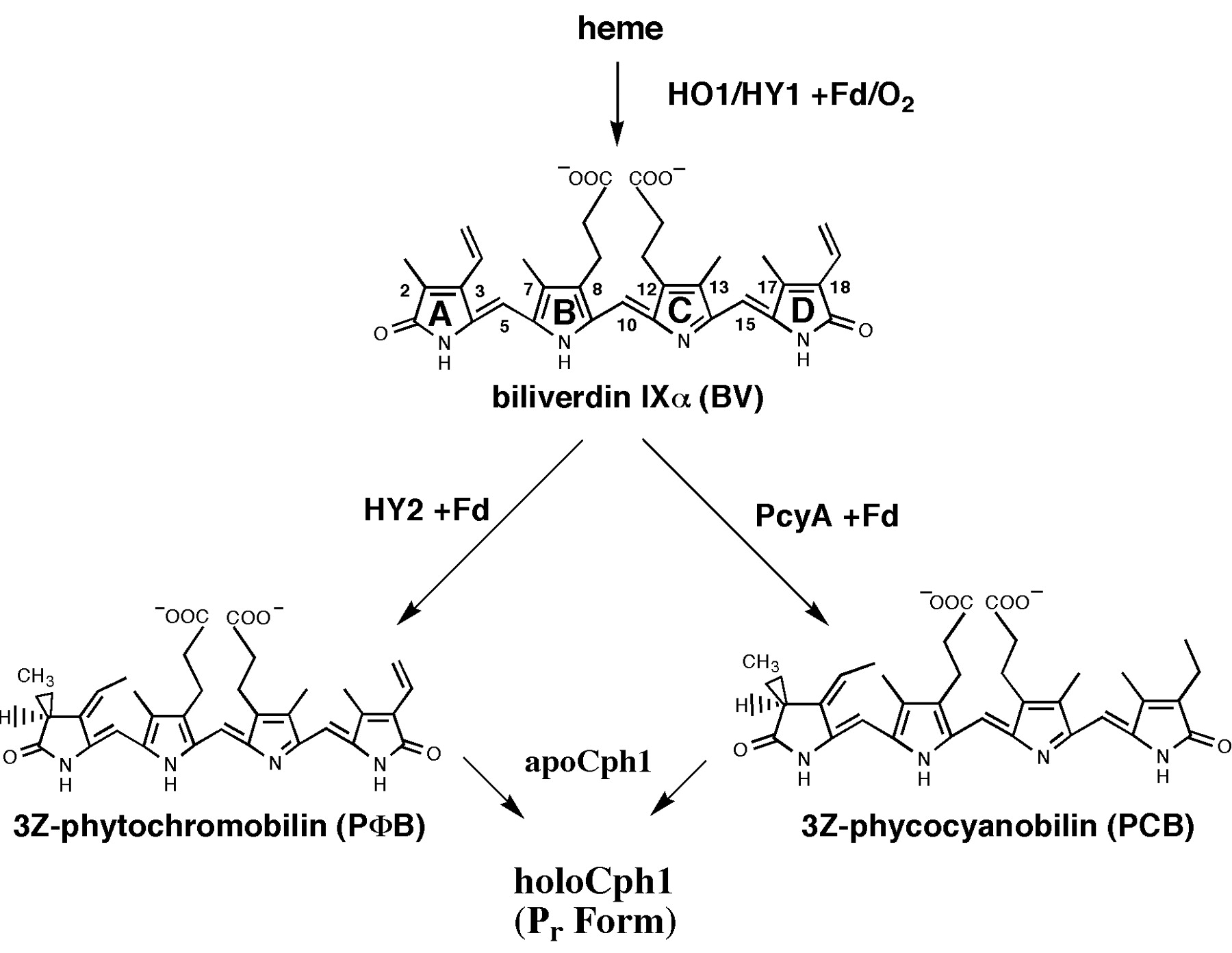
Biosynthesis of Phycocyanobilin
Phycocyanobilin undergoes a Z-E isomerization to its active form in case of red light and an E-Z isomerization to its inactive form in case of far-red light. The half-life of its active form Pfr is ~30 min, so continuous red light exposition is not necessary. A great advantage is that light-sensitive odorant and flavorings will not be destroyed. As phycocyanobilin is not naturally available in yeast one have to add the tetrapyrrole light-absorbing chromophore phycocyanobilin to the medium to get a functional light-switchable promoter system. But it also possible to bring the capability of phycocyanobilin synthesis in yeast by metabolic engineering. From heme, which is endogenous in yeast, there are only two steps of biosynthesis away from phycocyanobilin. The first step of phycocyanoblin is catalyzed by a heme oxygenase, the second step by a phycocyanobilin:ferredoxin oxidoreductase.
Induction Setup
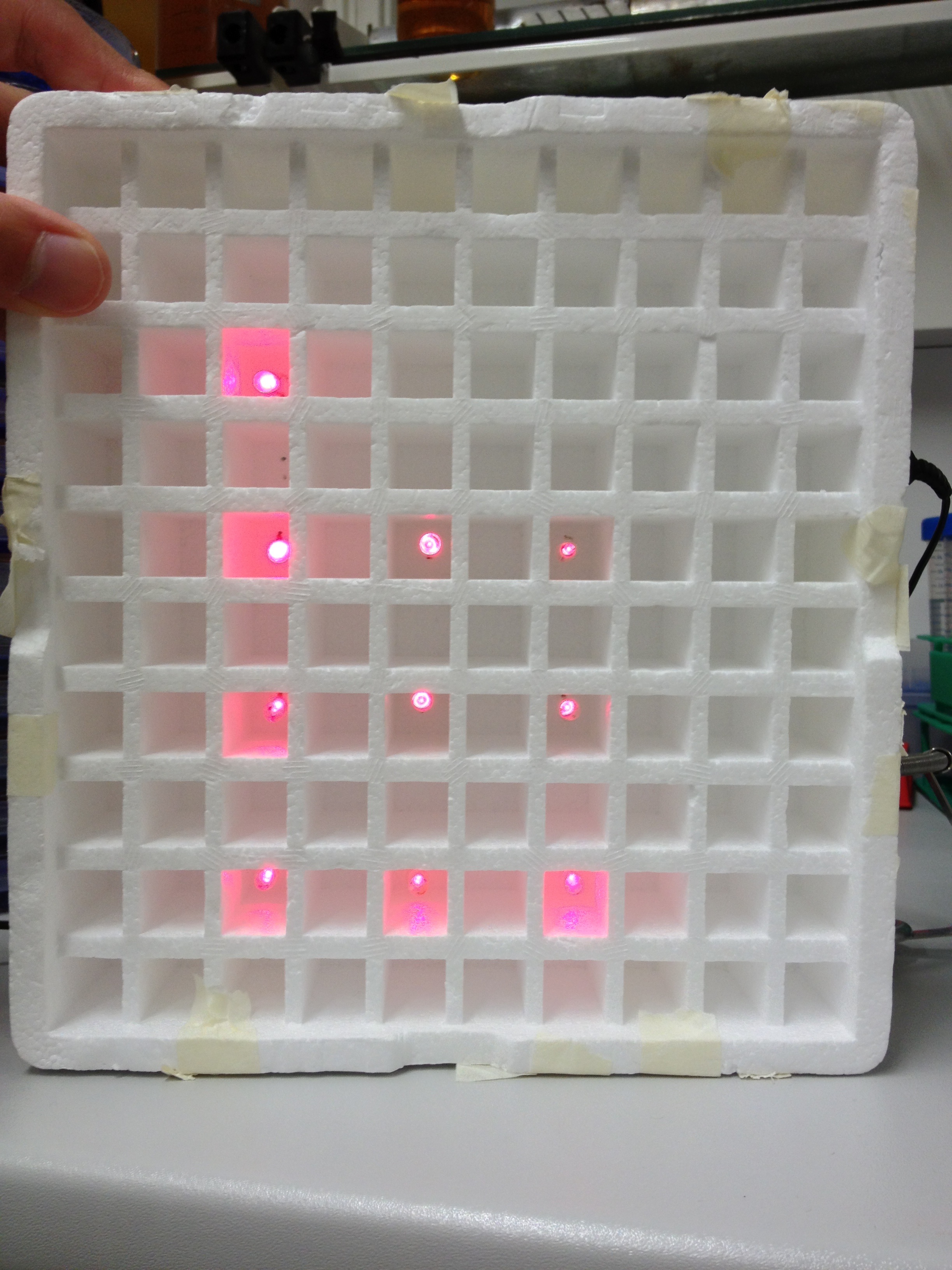
An array of 10 LEDs with emission peak at 660 nm [http://www.alldatasheet.com/datasheet-pdf/pdf/296270/ROITHNER/B5-436-30D.html] were attached into the molds of the packaging of 2 ml cuvettes and soldered together on the rear side of the packaging. As the cuvettes are the very ones that will later be used for illumination of the cells, the use of the packaging as LED matrix will allow quick removal during measurements and enhance accuracy of results.
Literature suggest pulsed illumination of the cells with a pulse duration of 10 seconds and a pulse frequency of 1 pulse every 5 minutes. The LEDs are actuated with an Arduino UNO micro-controller that puts the suggested protocol. The use of a micro-controller will allow us to easily test different pulse lengths and frequencies.
Results
Components of the Light-Switchable Promoter Systems
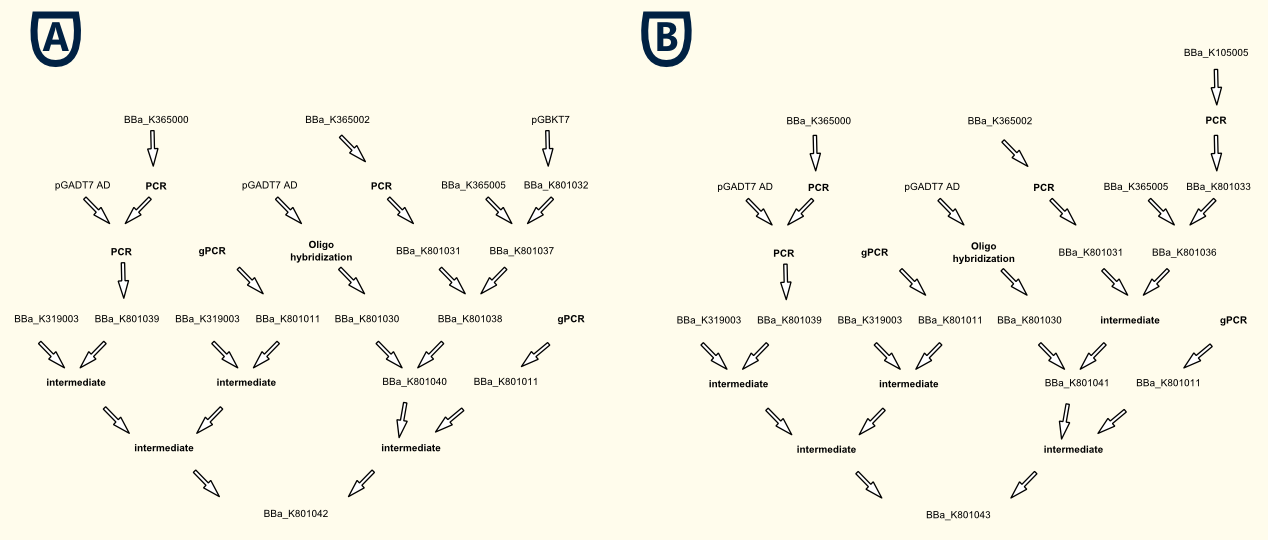
Two fusion proteins will be needed for a light-switchable promoter system. The first one is PIF3 fused to GAL4AD ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K801039 BBa_K801039]), the second one is GALDBD (GAL4 based) or LexA (LexA based) fused to PhyB ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K801040 BBa_K801040] or [http://partsregistry.org/wiki/index.php?title=Part:BBa_K801041 BBa_K801041]).
For PhyB and PIF3 we didn't used the whole protein coding sequence for our fusions. For PhyB we used the first 908 N-terminal amino acids which has been mapped to be sufficient for reversible photoconversion. Also for PIF3 only the first 100 N-terminal amino acids has been taken for our fusions due to the fact that they has been mapped to be only necessary for light-switchable binding to PhyB.
We successfully created all fusion proteins for a light-switchable promoter system based on GAL4 and LexA and even created a TEF1 promoter driven expression battery for all our components, for each type of the system (GAL4 and LexA based).
- Fusion protein for the first component (GAL4/LexA based):
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K801039 BBa_K801039: SV40NLS-GAL4AD-Linker-PIF3]
- Fusion protein for the second component (GAL4 based):
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K801040 BBa_K801040: SV40NLS-PhyB-Linker-GAL4DBD]
- Fusion protein for the second component (LexA based):
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K801041 BBa_K801041: SV40NLS-PhyB-Linker-LexA]
- TEF1 promoter driven gene expression battery for all parts of the GAL4 based light-switchable-promoter system:
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K801042 BBa_K801042: pTEF1_SV40NLS-GAL4AD-Linker-PIF3_tTEF1_pTEF1_SV40NLS-PhyB-Linker-GAL4DBD_tTEF1]
- TEF1 promoter driven gene expression battery for all parts of the LexA based light-switchable-promoter system:
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K801043 BBa_K801043: pTEF1_SV40NLS-GAL4AD-Linker-PIF3_tTEF1_pTEF1_SV40NLS-PhyB-Linker-GAL4LexA_tTEF1]
Extraction of PCB
Since there is no endogenous phycocyanobilin (PCB) in yeast, we have to add it to the medium first for our first proof-of-concept experiments. Later, we can implement the enzymes for the biosynthesis of phycocyanobilin ([http://partsregistry.org/wiki/index.php?title=Part:BBa_I15008 BBa_I15008] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K181000 BBa_K181000]) also in the finished gene expression batteries for our light-switchable promoter systems ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K801042 BBa_K801042] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K801043 BBa_K801043]).
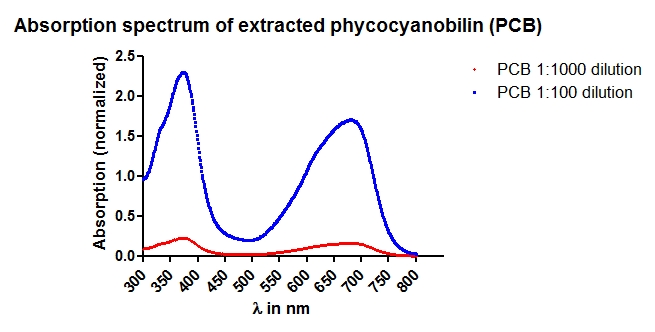
- Phycocyanobilin is extracted by methanolysis of dried Spirulina platensis. For detailed information please see our methods section
- The extracted phycocyanobilin is resuspended in DMSO and is kept at -20 °C until use.
- Absorption Spectrum for concentration determination.
Characterisation via Luciferase Assay
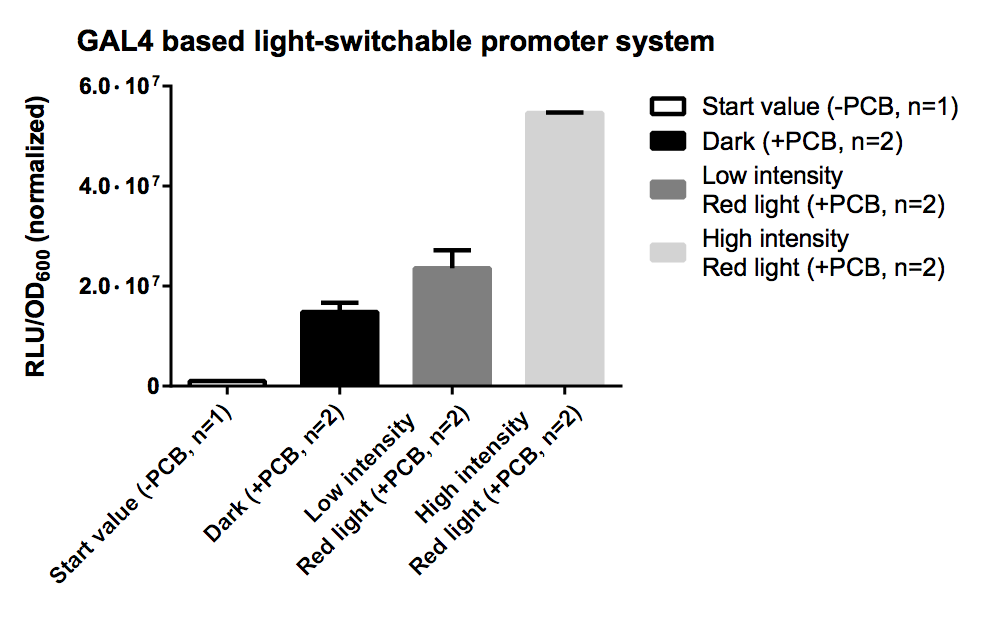
GAL4 Based System
PCB is necessary for correct folding of the PCB-PhyB-DNA-binding-site fusion protein, hence without PCB the output of our reporter system is close to zero.
As expression the promoter system was driven by the quite strong pTEF1 promoter and was transfected on a high copy vector http://www.ncbi.nlm.nih.gov/pubmed/6883512 Jayaram et al., 1983, there probably was a quite high level of both fusion proteins which led to unspecific binding and a quite high leaky transcription rate for the dark and low intensity samples. This problem could be dealt with by using a weaker promoter or a low copy vector.
The high intensity sample still shows a 3 fold increased induction for a 10 fold increased light intensity compared to the low intensity.
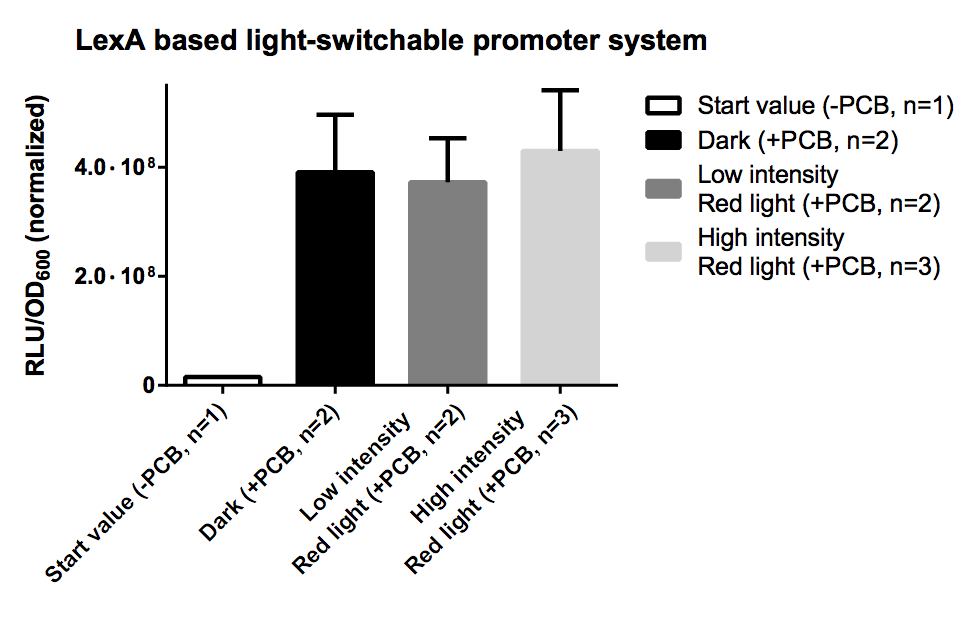
LexA Based System
[[File:TUM12 Dotplot LexA O+mCYC.jpg|thumb|right|400px|Fig. 9: Dotplot of http://partsregistry.org/wiki/index.php?title=Part:BBa_K165031 BBa_K165031], a minimal CYC1 promoter, preceded by LexA binding motifs. Dotplot reveals that the LexA motifs contain the typical sequence for a TATA-Box which dramatically rises the basal activity of the synthetic promoter construct, which explains the high basal activity of the LexA based LSPS.]
Again PCB is necessary for correct folding of the PCB-PhyB-DNA-binding-site fusion protein, hence without PCB the output of our reporter system is close to zero.
Here the expression the promoter system was as well driven by the quite strong pTEF1 promoter and was as well transfected on a high copy vector http://www.ncbi.nlm.nih.gov/pubmed/6883512 Jayaram et al., 1983, which again led to a high number of unspecific bindings of the fusion proteins. Overall the LexA repoter promoter seems to be more sensitive to the concentration of active transcription activating domains, which leads to virtually no difference between the dark, low intensity and high intensity samples. Furthermore investigation of the LexA promoter sequence showed that the LexA binding site [http://partsregistry.org/wiki/index.php?title=Part:BBa_K165031 BBa_K165031] itself contains four additional TATA boxes which explains the quite high basal expression rate.
Still the normalised RFU is about 10 fold higher than for the GAL4 based system so with a weaker promoter, a low copy plasmid and a different LexA recognition motif, this systems should be a better candidate for a light-switchable system.
Outlook for Further Reporter Systems
GAL4 Based Reporter Systems
For the GAL4 based light-switchable promoter system we have endogenous reporters in the Y190 S. cerevisiae strain.
The first one is an auxotrophic reporter for HIS3, an imidazoleglycerol-phosphate dehydratase, which catalyzes the sixth step in histidine biosynthesis. HIS3 is driven by a synthetic promoter with upstream GAL4 responsive elements. If plated on or inoculated in histidine deficient medium, there should be no growth of yeast, if they will be incubated in darkness or far-red light conditions. But under red light conditions the auxotrophy is reverted by expression of HIS3 due to the recruitment of GAL4AD through PhyB-PIF3 interaction.
The second reporter is LacZ, a beta-galactosidase, which will be controlled by pGAL1. Beta-galactosidase will be only expressed, if the light-switchable promoter system is switched on by red light.
LexA Based Reporter Systems
For the LexA based light-switchable promoter system we have to transfect yeast with a second plasmid coding for the reporter construct because there is no endogenous reporter system like for the GAL4 based system. Furthermore we didn't used the GAL4/GAL80 deletion strain Y190 in contrast to the GAL4 based system, since there is no need for the deletion because there is no interference between the prokaryotic LexA system the endogenous yeast signaling and the metabolism pathways.
Reference
- http://www.ncbi.nlm.nih.gov/pubmed/15823535 Chen et al., 2005 Chen, M., Tao, Y., Lim, J., Shaw, A., and Chory, J. (2005). Regulation of phytochrome B nuclear localization through light-dependent unmasking of nuclear-localization signals. Curr Biol, 15(7):637–42.
- http://www.ncbi.nlm.nih.gov/pubmed/19165330 Kikis et al., 2009 Kikis, E. A., Oka, Y., Hudson, M. E., Nagatani, A., and Quail, P. H. (2009). Residues clustered in the light-sensing knot of phytochrome B are necessary for conformer-specific binding to signaling partner PIF3. PLoS Genet, 5(1):e1000352.
- http://www.ncbi.nlm.nih.gov/pubmed/19749742 Levskaya et al., 2009 Levskaya, A., Weiner, O. D., Lim, W. A., and Voigt, C. A. (2009). Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature, 461(7266):997–1001.
- http://www.ncbi.nlm.nih.gov/pubmed/12355112 Mendelsohn, 2002 Mendelsohn, A. R. (2002). An enlightened genetic switch. Nat Biotechnol, 20(10):985–7.
- http://www.ncbi.nlm.nih.gov/pubmed/12219076 Shimizu-Sato et al., 2002 Shimizu-Sato, S., Huq, E., Tepperman, J. M., and Quail, P. H. (2002). A light-switchable gene promoter system. Nat Biotechnol, 20(10):1041–4.
- http://www.ncbi.nlm.nih.gov/pubmed/15486100 Khanna et al., 2004 Khanna, R., Huq, E., Kikis, E. A., Al-Sady, B., Lanzatella, C., and Quail, P. H. (2004). A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell, 16(11):3033–44.
- http://www.ncbi.nlm.nih.gov/pubmed/11553807 Gambetta and Lagarias, 2001 Gambetta, G. A. and Lagarias, J. C. (2001). Genetic engineering of phytochrome biosynthesis in bacteria. Proc Natl Acad Sci U S A, 98(19):10566–71.
- http://www.ncbi.nlm.nih.gov/pubmed/10466729 Ni et al., 1999 Ni, M., Tepperman, J. M., and Quail, P. H. (1999). Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature, 400(6746):781–4.
- http://www.ncbi.nlm.nih.gov/pubmed/12734586 Van Criekinge and Beyaert, 1999 Van Criekinge, W. and Beyaert, R. (1999). Yeast two-hybrid: State of the art. Biol Proced Online, 2:1–38.
- http://www.ncbi.nlm.nih.gov/pubmed/3891738 Wertman and Mount, 1985 Wertman, K. F. and Mount, D. W. (1985). Nucleotide sequence binding specificity of the LexA repressor of Escherichia coli K-12. J Bacteriol, 163(1):376–84.
- http://www.ncbi.nlm.nih.gov/pubmed/6883512 Jayaram et al., 1983 Jayaram, M., Li, Y. Y., and Broach, J. R. (1983). The yeast plasmid 2mu circle encodes components required for its high copy propagation. Cell, 34(1):95–104.
 "
"