Team:TU Munich/Notebook/Labjournal
From 2012.igem.org



June
Wednesday, June 13th
Exchange of the Multiple Cloning Site of pYES2
Investigator: Saskia, Daniela
Aim of the experiment: Exchange of the Multiple Cloning Site of pYES2
Hybridisation of the primers O1 with O2 and O3 with O4
Primer preparation:
- centrifugation
- dilution in the denoted quantity in bidest. water (concentration = 100 pmol/µl)
- centrifugation
Hybridisation:
| volume | reagent |
| 34 µl | ddH2O |
| 5 µl | PNK-buffer |
| 2.5 µl | Primer O1 |
| 2.5 µl | Primer O2 |
| 1 µl | PNK (10mM) |
| volume | reagent |
| 34 µl | ddH2O |
| 5 µl | PNK-buffer |
| 2.5 µl | Primer O3 |
| 2.5 µl | Primer O4 |
| 1 µl | PNK (10mM) |
- 30 min 37 °C
- 10 min 80 °C
- put the Thermo Block with the mixture in a styrofoam box and let it cool down over night
Thursday, June 14th
Exchange of the Multiple Cloning Site of pYES2
Investigator: Saskia, Daniela
Aim of the experiment: Exchange of the Multiple Cloning Site of pYES2
Digestion of pYES2 with HindIII and XbaI
| volume | reagent |
| 12 µl | Miniprep (pYES2 SH 1.7.3 with a concentration of 87.8 ng/µl) |
| 5 µl | NEB2 |
| 5 µl | 10x BSA |
| 2 µl | XbaI (10 U/µl) |
| 2 µl | HinIII (10U/µl) |
| 24 µl | ddH2O |
Incubation: 37 °C, 1.75 h
DNA preparative gel electrophoresis
- gel: 1% with LMP-agarose
- band 1: 10 µl DNA ladder (1kb)
- band 2: 50 µl probe + 5 µl loading dye
- 70 V, 90 min
Gelextration
- cut the bands (5.7-5.8 kb) and split it in two eppis
- m1=165.3 mg
- m2=211.1 mg
- QIAquick Gel Extractrion Kit
- eppi1: 495.9 µl QG-buffer
- eppi2: 633.3 µl QG-buffer
- step 6 was left out
- step 9: 30µl buffer, 4 min incubation
- the product was named P5
Transformation of plasmids from Prof. Schwab in E.coli XL-1 Blue
Investigator: Lara, Andrea
Aim of the experiment: Preparation of the plasmids for transformation
Overnight cultures of cells with limonenesynthase-plasmid from Prof. Schwab
- resuspend 50 µl / 200 µl of competent cells with 50 ml LB medium
- add 0,1 ml Ampicillin (100 µg/ml) and 0,28 ml Chloramphenicol (170 µ/ml) for strain 108
- add 0,07 ml Kanamycin (50 µg/ml) and 0,28 ml Chloramphenicol (170 µ/ml) for strain 106
- incubate at 37 °C
Friday, June 15th
Exchange of the Multiple Cloning Site of pYES2
Investigator: Saskia, Daniela
Aim of the experiment: Exchange of the Multiple Cloning Site of pYES2
Analytical DNA gel electrophoresis
- gel: 1.2 %
- band 1: 10 µl DNA ladder (1kb)
- band 2: 3 µl pYES2 digested + 7 µl TAE-buffer + 1 µl loading dye
- band 3: 3 µl O5 + 7 µl TAE-buffer + 1 µl loading dye
- band 4: 3 µl O6 + 7 µl TAE-buffer + 1 µl loading dye
- band 5: 10 µl DNA ladder (100 bp)
Ligation of Plasmid P5 (pYES2 digested) with the hybridized primers O5 and O6
| volume | reagent |
| 4 µl | pYES2 digested (P5) |
| 1 µl | O5 |
| 1 µl | O6 |
| 2 µl | T4-ligase buffer (10x) |
| 0,5 µl | T4 DNA-ligase |
| 11.5 µl | ddH2O |
Negative control
| volume | reagent |
| 4 µl | pYES2 digested (P5) |
| 2 µl | T4-ligase buffer (10x) |
| 0,5 µl | T4 DNA-ligase |
| 13.5 µl | ddH2O |
- water bath 16 °C
- after 3 h a probe for the transformation was taken
- the rest was ligated over the weekend
Transformation of E. coli with ligated products (P6)
- competent cells: SHXL1 Blue (by Simon)
- Transformation with
- ligation product (P6)
- negative control
results:
- P6 (100 µl): 1 clone
- P6 (concentrated): 30 clones
- negative control (100 µl): 0 clones
- negative control (concentrated): 6 clones
Transformation of plasmids from Prof. Schwab into E.coli XL-1 Blue
Investigator: Andrea
Aim of the experiment: Preparation of the plasmids for transformation
Determination of the concentration with Nano Drop
| Sample | concentration [ng/µl] |
| P3 | 1353 |
| P4 | no result |
- the strain 106 culture was not grown satisfying and were incubated for 2 more days
Miniprep of pGex-4T-1 of strain 108 from Prof. Schwab
- see QIAprep Spin Miniprep Kit
- stored as P3 (-20 °C)
Sunday, June 17th
Exchange of the Multiple Cloning Site of pYES2
Investigator: Saskia, Daniela
Aim of the experiment: Exchange of the Multiple Cloning Site of pYES2
Picking clones for Miniprep
- 10 clones of transformed E.coli with P6 were picked
- medium: 5ml LB with Amp
Monday, June 18th
Exchange of the Multiple Cloning Site of pYES2
Investigator: Saskia, Daniela
Aim of the experiment: Exchange of the Multiple Cloning Site of pYES2
Miniprep of transformed E.coli with P6
- QIAprepS Spin Miniprep Kit
- step 3: invert 2-3 times (don't shake to avoid destruction of genomic DNA)
- the 10 Minipreps were named: P7 - P16
Determination of the concentration with Nano Drop
| Sample | concentration [ng/µl] | 260/280 |
| P7 | 157.6 | 2.32 |
| P8 | 207.3 | 1.63 |
| P9 | 171.2 | 2.02 |
| P10 | 183.1 | 1.57 |
| P11 | 160.4 | 2.2 |
| P12 | 179.9 | 1.75 |
| P13 | 179.2 | 2.07 |
| P14 | 188.3 | 1.6 |
| P15 | 166.7 | 2.05 |
| P16 | 174.6 | 2.08 |
Controll digestion with HindIII XbaI and NgoMIV
- Samples P7-P16: 2.5 µl
- Negative controll pYES SH 1.7.3: 2.5 µl
- Master Mix HindIII and XbaI: 17,5 µl for a 20 µl preparation
| volume | reagent |
| 3 µl | HindIII |
| 3 µl | XbaI |
| 24 µl | NEB2 |
| 24 µl | 10x BSA |
| 156 µl | ddH2O |
- Master Mix NgoMIV: 17,5 µl for a 20 µl preparation
| volume | reagent |
| 6 µl | NgoMIV |
| 24 µl | NEB4 |
| 180 µl | ddH2O |
Incubation: 37 °C, 1.5 h
Analytical gel electrophoresis of P7-P16
- gel: 1.5 %
- gel 1:
- band 1: 10 µl DNA ladder (1 kb)
- band 2: 3 µl pYES2 SH 1.7.3 digested with HindIII and XbaI + 7 µl TAE buffer + 1 µl loading dye
- band 3 - 12: 3 µl P7-P16 digested with HindIII and XbaI + 7 µl TAE buffer + 1 µl loading dye
- gel 2:
- band 1: 10 µl DNA ladder (1 kb)
- band 2: 3 µl pYES2 SH 1.7.3 digested with NgoMIV + 7 µl TAE buffer + 1 µl loading dye
- band 3 - 12: 3 µl P7-P16 digested with NgoMIV + 7 µl TAE buffer + 1 µl loading dye
Tuesday, June 19th
Transformation of BBa_I742111 (Limonenesynthase) into E.coli XL-1 Blue
Investigator: Andrea
Aim of the experiment: Transformation
- For each Biobrick 100 µL cells were used and pooled together with 2 µL of plasmid DNA in a ERG on ice.
- Incubation on ice for 30 min.
- 5 min heat shock at 37 °C.
- Each ERG now is transferred in a new ERG prefilled with 1 mL of LB-medium and incubated in a cell-culture shaker at 37 °C for 45 min.
- 100 µL of these cell suspension were plated on antibiotic selection plates (Ampicillin for LexA and Kanamycin for heme oxygenase).
- The rest of the cell suspension is centrifuged at 13000 rpm for 60 s and the supernatant is discarded.
- The pellet is resuspended with 100 µL for each ERG and is plated on another antibiotic selection plate
- These 4 plates were put at 37 °C overnight
Wednesday, June 20th
Exchange of the Multiple Cloning Site of pYES2
Investigator: Saskia, Daniela
Aim of the experiment: Exchange of the Multiple Cloning Site of pYES2
Sequencing of P13 and P14: pYES2 with new MCS
- sequencing primer:
- 1.6 µM forward primer O9
- DNA P13 and P14
Transformation of BBa_I742111 (Limonenesynthase) into E.coli XL-1 Blue
Investigator: Daniela
Aim of the experiment: Transformation
Picking of Clones
- 6 clones were picked
- Incubation at 37 °C in LB + Amp
Thursday, June 21st
Transformation of BBa_I742111 and plasmids from Prof. Schwab into E.coli XL-1 Blue
Investigator: Lara, Andrea
Aim of the experiment: Controll of Transformation
Controll digestion
- Sample P3
| volume | reagent |
| 14 µl | Plasmid-DNA |
| 0,25 µl | NcoI |
| 2 µl | Buffer Tango (Fermentas) |
| 0,25 µl | HindIII |
| 2 µl | Buffer Red (Fermentas) |
| 1,5 µl | ddH2O |
- Sample P4
| volume | reagent |
| 6 µl | Plasmid-DNA |
| 0,25 µl | EcoRI |
| 2 µl | Buffer EcoRI (Fermentas) |
| 0,25 µl | NotI |
| 2 µl | Buffer Orange (Fermentas) |
| 9,5 µl | ddH2O |
- Sample Biobrick-clones
| volume | reagent |
| 5 µl | Plasmid-DNA |
| 0,25 µl | EcoRI |
| 2 µl | Buffer EcoRI (Fermentas) |
| 0,25 µl | PstI |
| 2 µl | Buffer Orange (Fermentas) |
| 10,5 µl | ddH2O |
Analytic Gelelectrophoresis
Friday, June 22nd
Transformation of E.coli XL1-Blue with pKS2µHyg-PAL-4Cl-CHS
Investigator: Ingmar, Volker
Aim of the experiment: Plasmid amplification
Operation Sequence:
- melting of 100 µl Ca-competent E.coli XL1-Blue cells
- addition of 1 µl of the Plasmid pKS2µHyg-PAL-4Cl-CHS
- incubation for 30 min on ice
- heat shock for 5 min at 37 °C
- transfer of cells to 1 ml LB-medium without antibiotics and incubate at 37°C and 180 rpm for 30 min
- plate 100 µl on an Amp-LB-plate
- sediment the leftover in a centrifuge (30 - 60 sec, 13 000 rpm) and resuspend the sediment in 100 µl LB-medium and plate it as well on an Amp-LB-plate
Saturday, June 23rd
Quick Change mutagenis to remove NgoMIV from pYES2
Investigator: Ingmar, Volker
Aim of the experiment: Generation of an RFC 25 compatible version of the pYes2 Vector.
PCR
Reaction batch
| volume | reagent |
| 2.5 µl | 10x Pfu Ultra II buffer |
| 4 µl | Plasmid P7 pYes2_RFC25 MCS 1.1 template |
| 0.5 µl | 1:10 dilution of O38 (10 pmol/µL) |
| 0.5 µl | 1:10 dilution of O39 (10 pmol/µL) |
| 17 µl | ddH2O |
| 0.5 µl | dNTP mix |
| 0.5 µl | Pfu Turbo DNA polymerase (2.5 U / µl) |
PCR cycling parameters
| Segment | Cycles | Temperature | Time |
| 1 | 1 | 95 °C | 30 sec |
| 2 | 15 | 95°C | 30 sec |
| 55°C | 1 min | ||
| 68°C | 6 min |
- The vector resulting from the PCR-product was named pYes2_RFC25 MCS 1.2.
- Digestion of the parental DNA with DpnI: Addition of 1 µl DpnI to the PCR batch and incubate for 1 h at 37 °C.
Transformation into E.coli Xl1-Blue Operation Sequence
- melting of 100 µl Ca-competent E.coli XL1-Blue cells
- addition of 1 µl of the Plasmid P7 pYes2_RFC25 MCS 1.2
- incubation for 30 min on ice
- heat shock for 5 min at 37 °C
- transfer of cells to 1 ml LB-medium without antibiotics and incubate at 37°C and 180 rpm for 30 min
- plate 100 µl on an Amp-LB-plate
- sediment the leftover in a centrifuge (30 - 60 sec, 13 000 rpm) and resuspend the sediment in 100 µl LB-medium and plate it as well on an Amp-LB-plate
Sunday, June 24th
Miniprep of E.coli XL1-Blue with pKS2µHyg-PAL-4Cl-CHS
Investigator: Ingmar, Volker
Aim of the experiment: Plasmid purification
Operation Sequence:
- A single clone of E.coli XL1-Blue with pKS2µHyg-PAL-4Cl-CHS was picked an transferred to 5 ml LB Amp on saturday evening. Incubation overnight at 37°C 180 rpm.
- Using a Quiagen kit a miniprep of the overnight culture was done.
Quick Change mutagenis to remove NgoMIV from pYES2
Investigator: Ingmar, Volker
Aim of the experiment: Removal of a NgoMIV restriction site in the backbone of pYes2.
Operational sequence:
- A single clone of E. coli pYes2_RFC25 MCS 1.2 was picked an transferred to 5 ml LB Amp. Incubation overnight at 37°C 180 rpm.
Transformation of 2 Biobricks into E. coli XL1-Blue
Investigator: Jeffery Truong
Aim of the experiment: Transformation of Biobricks into E. coli for plasmid propagation for PCR with new RFC pre- and suffix primer in order to do protein fusions.
- 2 Biobricks from the distribution kit were used: First, LexA (BBa_K105005, Plate 3 Well 9E) in the pSB1A2 plasmid with ampicillin resistance and second, the heme oxygenase (BBa_I15008, Plate 2 Well 13J) in the pSB2K3 plasmid with kanamycin resistance.
- 10 µL of autoclaved H2O were added to each well on the distribution kit. The well immediately turned red which means that one does it right.
- The now resuspended DNA liquids were transferred into a new ERG on ice.
- CaCL2 competent E. coli XL1-Blue cells from the stock were gently defrezed on ice.
- For each Biobrick 100 µL cells were used and pooled together with 2 µL of plasmid DNA in a ERG on ice.
- Incubation on ice for 30 min.
- 5 min heat shock at 37 °C.
- Each ERG now is transferred in a new ERG prefilled with 1 mL of LB-medium and incubated in a cell-culture shaker at 37 °C for 45 min.
- 100 µL of these cell suspension were plated on antibiotic selection plates (Ampicillin for LexA and Kanamycin for heme oxygenase).
- The rest of the cell suspension is centrifuged at 13000 rpm for 60 s and the supernatant is discarded.
- The pellet is resuspended with 100 µL for each ERG and is plated on another antibiotic selection plate
- These 4 plates were put at 37 °C overnight
Monday, June 25th
Miniprep of E. coli XL1-Blue with pYes2_RFC25 MCS 1.2
Investigator: Alois, Martin
Aim of the experiment: proof of successful removal of NgoMIV in the backbone of pYes2
Operation Sequence:
- Mini prep of pYes2 1.2. The resulting purified DNA is P33.
- Control digest of pYes2_RFC25 MCS 1.2 and p13 (+ analytical gel electrophoresis: 90 V, 1 h:
* 15 µl ddH20 * 2 µl NEBuffer4 * 0,5 µl NgoMIV * 2,5 µl pYes2 1.2/p13 * 37°C, 1 h.
Quick Change mutagenis to remove SpeI from pYES2_RFC25 MCS 1.2
Investigator: Ingmar, Volker
Aim of the experiment: Generation of an RFC 25 compatible version of the pYes2 Vector.
PCR
Reaction batch
| volume | reagent |
| 2.5 µl | 10x Pfu Ultra II buffer |
| 4 µl | Plasmid P33 template |
| 0.5 µl | 1:10 dilution of O44 (10 pmol/µL) |
| 0.5 µl | 1:10 dilution of O45 ((10 pmol/µL) |
| 17 µl | ddH2O |
| 0.5 µl | dNTP mix |
| 0.5 µl | Pfu Turbo DNA polymerase (2.5 U / µl) |
PCR cycling parameters
| Segment | Cycles | Temperature | Time |
| 1 | 1 | 95 °C | 30 sec |
| 2 | 15 | 95°C | 30 sec |
| 55°C | 1 min | ||
| 68°C | 6 min |
- The procedure was furthermore applied to P13 and P14.
- The vector resulting from the PCR-product was named pYes2_RFC25 MCS 1.3.
- Digestion of the parental DNA with DpnI: Addition of 1 µl DpnI to the PCR batch and incubate for 1 h at 37 °C.
Transformation into E.coli Xl1-Blue Operation Sequence
- This operation sequence was applied to the PCR prducts of P33, P13 and P14 respectively.
- melting of 100 µl Ca-competent E.coli XL1-Blue cells
- addition of 1 µl of the PCR product
- incubation for 30 min on ice
- heat shock for 5 min at 37 °C
- transfer of cells to 1 ml LB-medium without antibiotics and incubate at 37°C and 180 rpm for 30 min
- plate 100 µl on an Amp-LB-plate
- sediment the leftover in a centrifuge (30 - 60 sec, 13 000 rpm) and resuspend the sediment in 100 µl LB-medium and plate it as well on an Amp-LB-plate
Picking of E. coli cells on antibiotic selection plates: pSB1A2 plasmid with BBa_K105005 (LexA) and pSB2K3 plasmid BBa_I15008 (heme oxygenase)
Investigator: Jeffery Truong
Aim of the experiment: Picking colonies from transformed E. coli XL1-Blue, 4x picked for each Biobrick.
- pSB1A2 plasmid with BBa_K105005 (LexA): Colonies were on both ampicillin selection plates, the one with diluted cell suspension and the one with concentrated E. coli cell suspension. Typical E. coli colony morphology. Picking was performed on the plate with diluted cell suspension.
- pSB2K3 plasmid BBa_I15008 (heme oxygenase): Colonies were only on the kanamycin selection plate with concentrated cell suspension. The one with diluted susepension was empty. Typical but very small E. coli colonies. Picking was performed from the first plate.
- Picked pipette tips was transferred into a special cell-culture tubes with air-permeable, but sterile cover. In each tube 4 mL of LB-medium + ampicillin (???)(for pSB1A2) or kanamycin (35 mg/mL) (for pSB2K3).
- 4 colonies for each Biobrick was picked; total: 8 tubes overnight culture.
- These tubes were transferred in a cell culture shaker at 37 °C and were incubated overnight
Tuesday, June 26th
Quick Change mutagenis to remove SpeI from pYes2_RFC25 MCS
Investigator: Ingmar
Aim of the experiment: Removal of a SpeI restriction site in the backbone of pYes2.
Operational sequence:
- For each transformation of the PCR-products of P14 and P33 a single clone was picked an transferred to 6 ml LB Amp. Incubation overday at 37°C 180 rpm. The transfomation with the PCR product of P13 was not successfull. Therfore no clone could be picked.
- Using a Quiagen kit a miniprep of the overnight culture was done.
- The resulting purified DNA was aliquoted in new tubes labeled as follows:
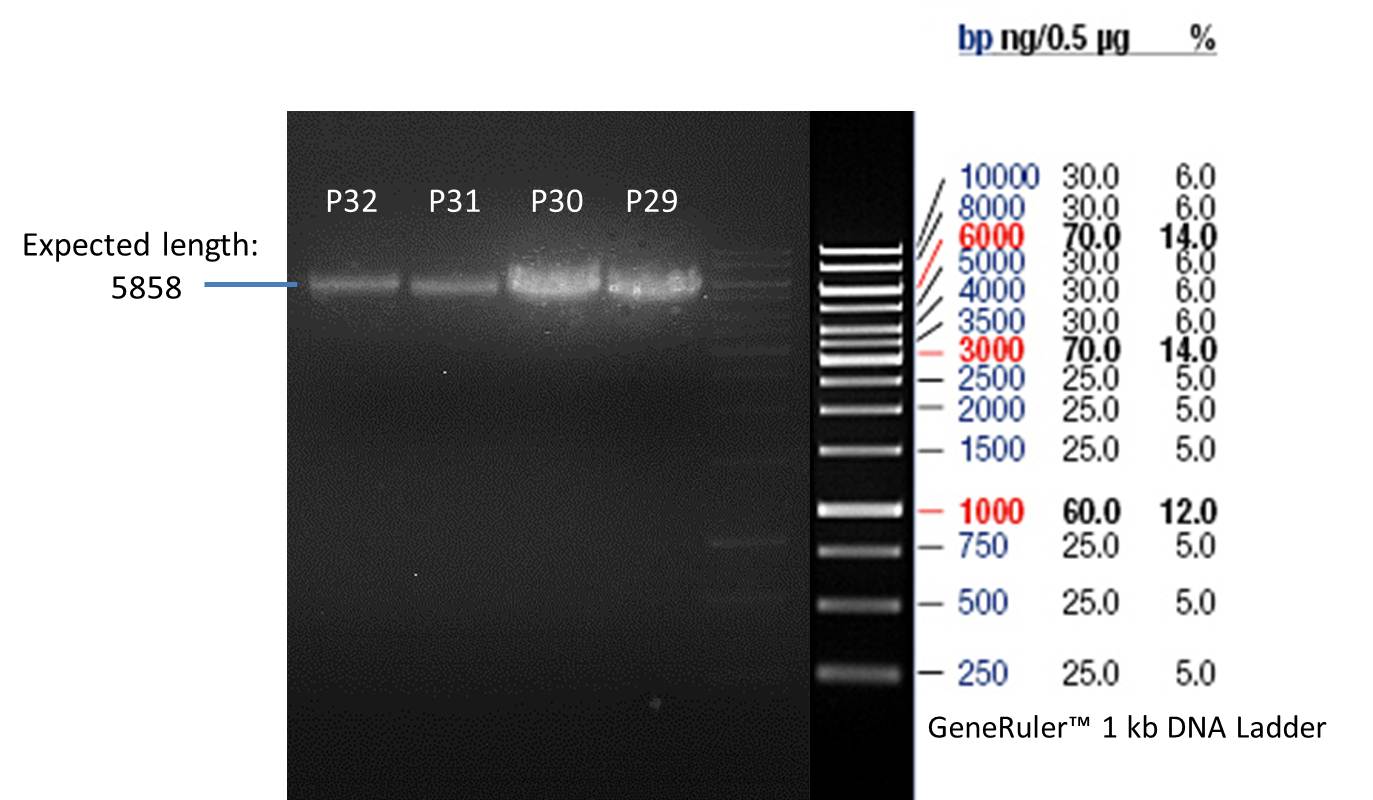
PCR product of P33(transformation done by Ingmar): P29
PCR product of P33(transformation done by Saskia&Jara): P30
PCR product of P14(transformation done by Ingmar): P31
PCR product of P14(transformation done by Saskia&Jara): P32
- Afterwards a control digestion of P29-P32 was done.
Reaction batch
| Plasmid | P29 | P30 | P31 | P32 |
| NEB4 buffer | 2 µl | 2 µl | 2 µl | 2 µl |
| DNA | 2,5 µl | 2,5 µl | 5 µl | 5 µl |
| SpeI-HF | 0.25 µl | 0.25 µl | 0.25 µl | 0.25 µl |
| NgoMIV | 0.25 µl | 0.25 µl | ||
| ddH2O | 15 µl | 15 µl | 12.75 µl | 12.75 µl |
| Sum | 20 µl | 20 µl | 20 µl | 20 µl |
- Incubation at 37 °C for 1h.
- Verification of control digest by agarose gel electrophoresis:
20 µl of each digest product was mixed with 2 µl DNA loading buffer and loaded into the gel. The separation process lasted 1h at 90 V.
Verification of the PCR products P30, P31 and P33
Investigator: Saskia, Jara
Aim of the experiment: Verification of the PCR produts P30, P31 and P33
Nano Drop
| Sample | concentration [ng/µl] | 260/280 |
| P33 | 1072.6 | 1.01 |
| P30 | 1588 | 1.28 |
| P31 | 926.2 | 0.82 |
Analytical gel electrophoresis
- gel: 1 %
- band 1: 10 µl DNA ladder (1kb)
- band 2: P30
- band 3: P33
- band 4: P31
Control of the competent cells and transformation with P20
Investigator: Saskia, Jara
Aim of the experiment: Control of the competent cells and transformation with P20 Transformation
- competent cells: by Simon and Ingmar
- plasmid: P20
result:
- successful transformation: red colonies
PCR of PAL, 4CL, CHS, OMT (Coumaryl-CoA)
Investigator: Daniela, Mary
Determination of concentration of plasmids (Nanodrop): c(pKS2µHyg-PAL-4CL-CHS) = 500 ng/µl c (pOMT) = 20 ng/µl
PCR:
| Name of tube | Enzyme | consensus (+)/ consensless (-) | used Oligos |
| CHS - | CHS | - | O13 and O24 |
| CHS + | CHS | + | O23 and O24 |
| PAL - | PAL | - | O15 and O16 |
| PAL + | PAL | + | O22 and O16 |
| OMT - | OMT | - | O17 and O26 |
| OMT + | OMT | + | O25 and O26 |
| 4CL - | 4CL | - | O19 and O20 |
| 4CL + | 4CL | + | O21 and O20 |
Reaction batch
| volume | reagent |
| 5 µl | 10x Pfu Ultra II buffer |
| 4 µl | dNTP's (each 2.5 mM) |
| 0.5 µl | Pfu Ultra II (2.5 U/µL) |
| 5 µl | 1:10 dilution of used forward primers (10µM) |
| 5 µl | 1:10 dilution of used reversed primers (10µM) |
| 1 µl | DNA (pKS2µHyg-PAL-4CL-CHS 50 ng/µL or pOMT 20 ng/µL) |
| 29.5 µL | bidest. sterile Water |
PCR cycling parameters
| Segment | Cycles | Temperature | Time |
| 1 | 1 | 95 °C | 5 min (and adding Pfu Ultra after 3 min) |
| 2 | 30 | 95°C | 30 sec |
| 46°C | 2.5 min | ||
| 72°C | 1.5 min | ||
| 3 | 72°C | 5 min |
PCR purification
- Purification was done using QIAquick PCR Purification Kit (250)
Analytical Gel Electrophoresis:
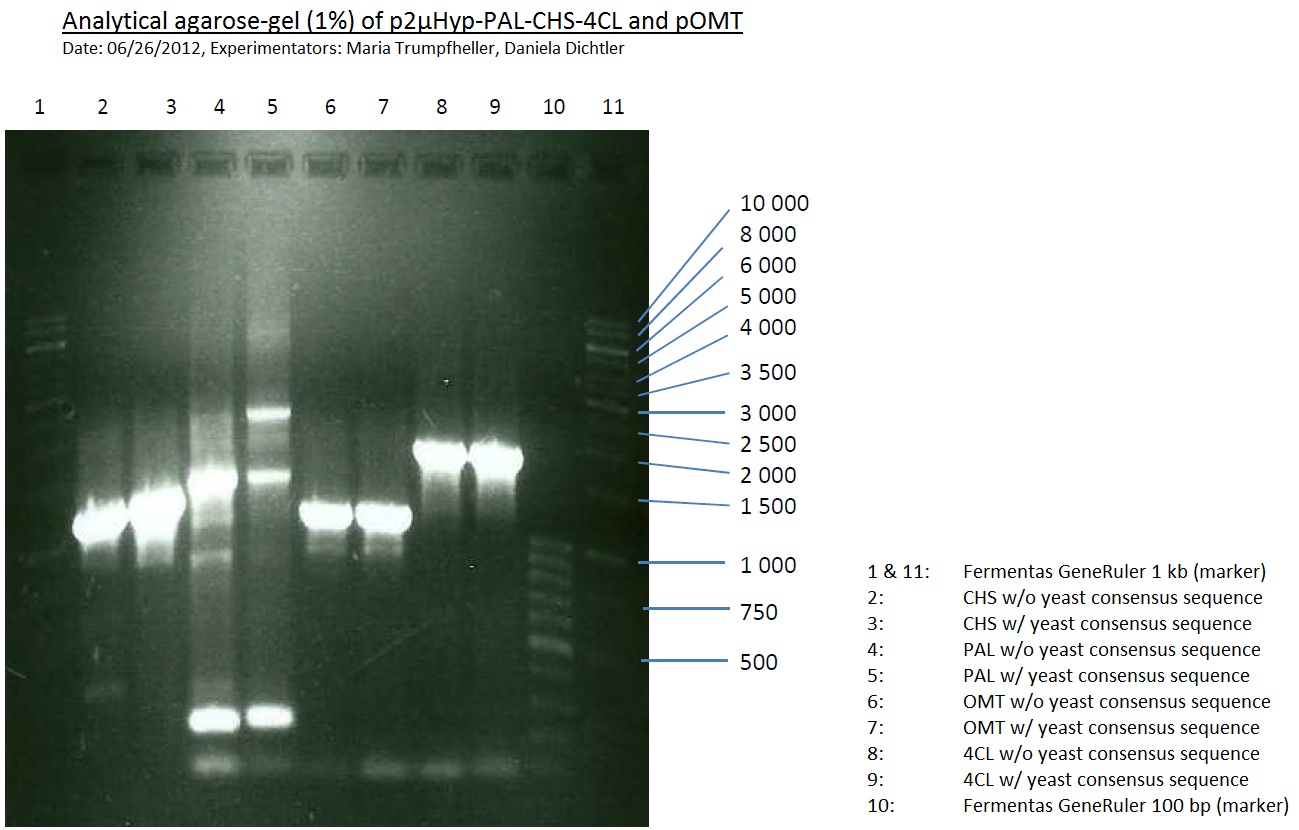
-> going on with CHS, 4CL and OMT; the PCR of PAL will be repeated
Miniprep and analytical gel of picked transformed overnight culture with pSB1A2 plasmid with BBa_K105005 (LexA) pSB2K3 plasmid BBa_I15008 (heme oxygenase)
Investigator: Jeffery Truong, Georg Schützinger
Aim of the experiment: Plasmid isolation from the picked transformed overnight E. coli cells with pSB1A2 plasmid with BBa_K105005 (LexA) pSB2K3 plasmid BBa_I15008 (heme oxygenase).
- The LB-medium with antibiotics of every tube was opaque which means that the picked cells were successfully inoculated.
- Centrifugation step at 5000 rpm for 10 min at 16 °C.
- Every single step now was performed on ice.
- Miniprep (Qiagen Qiaprep spin) after manufacturer's protocoll.
- Analytical restriction master mix was prepared after following scheme (Using XbaI and PstI):
- 4.4 µL XbaI
- 4.4 µL PstI
- 17.6 µL Tango-buffer (10x)
- 132 µL ELGA H2O
- 17.5 µL from the master mix was poooled together with 2.5 µL of plasmid DNA. That corresponds to 2.5 µL of plasmid DNA, 0.25 µL XbaI, 0.25 µL PstI, 2 µL Tango-buffer (10x), 15 µL ELGA H2O.
- Incubation at 37 °C for 120 min on a ERG heating unit.
- BUT: error was performed during preparing the digested plasmid DNA for analytical gelelectrophoresis in the dilution step. 1:10 dilution of analyctical probe with DNA loading buffer:
- 3.3 µL sample (contains already 1x loading buffer!)
- 0.7 µL loading buffer (?x)
- 6 µL TAE-buffer
- Should have done: 3 µL sample + 1 µL loading buffer (10x) + 6 µL TAE-buffer (1x)
- DNA-ladder preperation: 10 µL ladder stock solution + 10 µL DNA loading buffer + 80 µL TAE-buffer. 10 µL of this solution was pipetted in one gel pocket of the prepared 1% agarose gel including ehtiudiumbromid.
- 20 µL of each samples were also pipetted into the gel pockets.
- The gel pockets were pipetted after following scheme:
| Heme oxygenase (colony 1) | Heme oxygenase (colony 2) | Heme oxygenase (colony 3) | Heme oxygenase (colony 4) | DNA-ladder | LexA (colony 1) | LexA (colony 2) | LexA (colony 3) | LexA (colony 4) |
- Gel electrophoresis at 90 V
- After 20 min the resolution was still poor; 20 min longer.
- Analytical Gel okay, but samples interchanged
Friday, June 29th
Preparative digest of PCR-products of 4CL, CHS and OMT
Investigator: Katrin, Mary
each digestion will dure 2.5h at 37°C
- CHS: digestion with Xba1 and HF-Age1 (both NEB)
| volume | reagent |
| 25µl | PCR-product |
| 5µl | Buffer NEB4 |
| 0.5µl | BSA |
| 1µl | Xba1 (NEB; 20u/µl) |
| 1µl | HF-Age1 (NEB; 20u/µl) |
| 17.5µl | bidest. sterile H2O |
- OMT: digestion with Xba1 and HF-Age1 (both NEB)
| volume | reagent |
| 25µl | PCR-product |
| 5µl | Buffer NEB4 |
| 0.5µl | BSA |
| 1µl | Xba1 (NEB; 20u/µl) |
| 1µl | HF-Age1 (NEB; 20u/µl) |
| 17.5µl | bidest. sterile H2O |
- 4CL: digestion with Xba1 and Pst1 (both Fermentas)
| volume | reagent |
| 25µl | PCR-product |
| 5µl | Buffer Tango |
| 2µl | Xba1 (Fermentas; 10u/µl) |
| 3µl | Pst1 (Fermentas; 10u/µl) |
| 15µl | bidest. sterile H2O |
Preparative Gelelectrophoresis of PCR-products of 4CL, CHS
Investigator: Katrin, Mary
picture follows!
Gelextraction of 4CL+, 4CL-, CHS+, CHS- (bands are as expected; +=with consensus-sequence, -=without consensus-sequence)
DNA-purification with Kit from Quiagen
Quick Change mutagenesis to remove PstI in URA3 from pYES2_RFC25 MCS 1.2
Investigator: Ingmar
Aim of the experiment: Generation of an RFC 25 compatible version of the pYes2 Vector.
PCR
Reaction batch
| volume | reagent |
| 2.5 µl | 10x Pfu Ultra II buffer |
| 4 µl | Plasmid P29 template |
| 0.5 µl | 1:10 dilution of O40 (10 pmol/µL) |
| 0.5 µl | 1:10 dilution of O41 ((10 pmol/µL) |
| 17 µl | ddH2O |
| 0.5 µl | dNTP mix |
| 0.5 µl | Pfu Turbo DNA polymerase (2.5 U / µl) |
PCR cycling parameters
| Segment | Cycles | Temperature | Time |
| 1 | 1 | 95 °C | 30 sec |
| 2 | 15 | 95°C | 30 sec |
| 55°C | 1 min | ||
| 67°C | 6.5 min |
- The procedure was furthermore applied to P31.
- Digestion of the parental DNA with DpnI: Addition of 1 µl DpnI to the PCR batch and incubate for 1 h at 37 °C.
Transformation into E.coli Xl1-Blue Operation Sequence
- This operation sequence was applied to the PCR prducts of P29 and P31 respectively.
- melting of 100 µl Ca-competent E.coli XL1-Blue cells on ice
- addition of 1 µl of the PCR product
- incubation for 30 min on ice
- heat shock for 5 min at 37 °C
- transfer of cells to 1 ml LB-medium without antibiotics and incubate at 37°C and 180 rpm for 30 min
- plate 100 µl on an Amp-LB-plate
- sediment the leftover in a centrifuge (30 - 60 sec, 13 000 rpm) and resuspend the sediment in 100 µl LB-medium and plate it as well on an Amp-LB-plate
Saturday, June 30th
Quick Change mutagenis to remove PstI in the URA 3 gene from pYes2_RFC25 MCS
Investigator: Ingmar
Aim of the experiment: Removal of a PstI restriction site in the backbone of pYes2.
Operational sequence:
- For each transformation of the PCR-products of P29 and P30 a single clone was picked an transferred to 6 ml LB Amp. Incubation overnight at 37°C 180 rpm. The transfomation with the PCR product of P31, P32 and P33 was not successfull. Therfore no clone could be picked from these plates and four instead of one clone was picked from the plates containing the transformations of P29.
July
Sunday, July 1st
Quick Change mutagenis to remove PstI in the URA 3 gene from pYes2_RFC25 MCS
Investigator: Ingmar
Aim of the experiment: Removal of a PstI restriction site in the backbone of pYes2.
Operational sequence:
- Using a Quiagen kit a miniprep of the overnight culture was done.
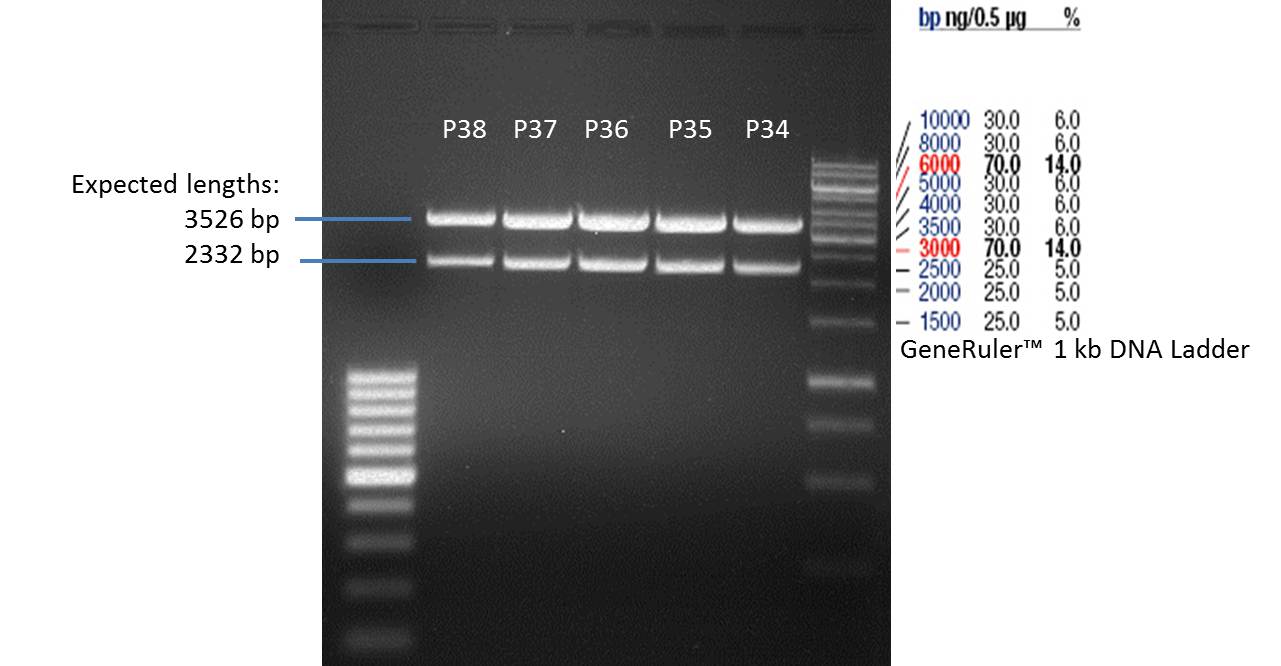
- The resulting purified DNA was aliquoted in new tubes labeled as follows:
1st PCR product of P29: P34
2nd PCR product of P29: P35
3rd PCR product of P29: P36
4th PCR product of P29: P37
PCR product of P30: P38
- Afterwards a control digestion of P34-P38 was done.
Reaction batch
| Plasmid | P34 | P35 | P36 | P37 | P38 |
| Fermentas 10x R buffer | 0.5 µl | 0.5 µl | 0.5 µl | 0.5 µl | 0.5 µl |
| DNA | 2 µl | 2 µl | 2 µl | 2 µl | 5 µl |
| PstI | 0.25 µl | 0.25 µl | 0.25 µl | 0.25 µl | 0.25 µl |
| ddH2O | 17.25 µl | 17.25 µl | 17.25 µl | 17.25 µl | 14.25 µl |
| Sum | 20 µl | 20 µl | 20 µl | 20 µl | 20 µl |
- Incubation at 37 °C for 1h.
- Verification of control digest by agarose gel electrophoresis:
20 µl of each digest product was mixed with 4 µl 6x DNA loading buffer and loaded into the gel. The separation process lasted 1h at 90 V.
- All digest products show the expected two bonds at 3526 bp and at 2332 bp. The Miniprep product P35 was chosen to be used for the further Quickchange Mutagenesis.
Quick Change mutagenesis to remove PstI in the 2µ ori from pYES2_RFC25 MCS
Investigator: Ingmar
Aim of the experiment: Generation of an RFC 25 compatible version of the pYes2 Vector.
PCR
Reaction batch 1
| volume | reagent |
| 2.5 µl | 10x Pfu Ultra II buffer |
| 4 µl | Plasmid P35 template |
| 0.5 µl | 1:10 dilution of O42 (10 pmol/µL) |
| 16.5 µl | ddH2O |
| 0.5 µl | dNTP mix |
| 0.5 µl | Pfu Turbo DNA polymerase (2.5 U / µl) |
Reaction batch 2
| volume | reagent |
| 2.5 µl | 10x Pfu Ultra II buffer |
| 4 µl | Plasmid P35 template |
| 0.5 µl | 1:10 dilution of O43 (10 pmol/µL) |
| 16.5 µl | ddH2O |
| 0.5 µl | dNTP mix |
| 0.5 µl | Pfu Turbo DNA polymerase (2.5 U / µl) |
PCR cycling parameters
| Segment | Cycles | Temperature | Time |
| 1 | 1 | 95 °C | 30 sec |
| 2 | 10 | 95°C | 30 sec |
| 55°C | 1 min | ||
| 67°C | 6 min |
- Having completed the PCR cycling parameters listed above both PCR reaction batches were mixed together and the cycling parameters listed above were one time more applied.
- Digestion of the parental DNA with DpnI: Addition of 1 µl DpnI to the PCR batch and incubate for 1 h at 37 °C.
Transformation into E.coli Xl1-Blue Operation Sequence
- melting of 100 µl Ca-competent E.coli XL1-Blue cells on ice
- addition of 1 µl of the PCR product
- incubation for 30 min on ice
- heat shock for 5 min at 37 °C
- transfer of cells to 1 ml LB-medium without antibiotics and incubate at 37°C and 180 rpm for 30 min
- plate 100 µl on an Amp-LB-plate
- sediment the leftover in a centrifuge (30 - 60 sec, 13 000 rpm) and resuspend the sediment in 100 µl LB-medium and plate it as well on an Amp-LB-plate
Monday, July 2nd
Repetition of PCR of PAL
Investigator: Mary
Reaction batch
| volume | reagent |
| 5 µl | 10x Pfu Ultra II buffer |
| 4 µl | dNTP's (each 2.5 mM) |
| 0.5 µl | Pfu Ultra II (2.5 U/µL) |
| 5 µl | 1:10 dilution of used forward primers (10µM) |
| 5 µl | 1:10 dilution of used reversed primers (10µM) |
| 1 µl | DNA (pKS2µHyg-PAL-4CL-CHS 50 ng/µL) |
| 29.5 µL | bidest. sterile Water |
PCR cycling parameters
| Segment | Cycles | Temperature | Time |
| 1 | 1 | 95 °C | 2 min (and adding Pfu Ultra after 2 min) |
| 2 | 30 | 95°C | 30 sec |
| 55°C | 1 min | ||
| 72°C | 2.5 min | ||
| 3 | 72°C | 5 min |
PCR of LexA with primers including the RFC25 pre- and suffix
Investigator: Jeffery Truong, Georg Schützinger
Aim of the experiment: The Biobrick BBa_K105005 (LexA) has a RFC10 pre- and suffix, but we need RFC25 pre- and suffix for protein fusion, so we have to do a PCR with primer containing the RFC25 pre- and suffix.
- The received forward and reverse primer TUM12-LexA-fw and TUM12-LexA-rv are resuspended in 204 µL and 221 µL ELGA water as described in the data sheet to get a final primer concentration of 100 pmol/µL=100 µM. For the PCR reaction mixture we took 0.5 µL of these resuspended primer and add 4.5 µL of ELGA water to get a final primer concentration of 10 µM.
- Clone 3 of BBa_K105005 (LexA) has beed choosen for the PCR (ERG No. P23).
PCR reaction mixture
| volume | reagent |
| 10 µl | 5x OneTaq Standard Reaction Buffer |
| 1 µl | 10 mM dNTPs |
| 1 µl | 10 µM Forward Primer |
| 1 µl | 10 µM Reverse Primer |
| 0.25 µL | OneTaq Hot Start DNA Polymerase (Finally: 1.25 units/50 µL) |
| 1 µl | Plasmid DNA (BBa_K105005) from P23 (Clone 3) |
| 35.75 µL | ELGA Water |
| =50 µL | TOTAL |
- The PCR program was performed after following scheme:
| Initial denaturation | 94 °C | 30 s |
| 30 cycles | 94 °C | 30 s |
| 55 °C | 60 s | |
| 68 °C | 60 s | |
| Final extension | 68 °C | 5 min |
| Hold | 4 °C | overnight |
Tuesday, July 3rd
Analytic gelelectrophoresis of cleaned up PCR product from LexA with primer containing RFC25 pre- and suffix
Investigator: Jeffery Truong
Aim of the experiment: Analytical gelelectrophoresis of cleaned up PCR product from LexA (BBa_K105005) with primer containing the RFC25 pre- and suffix (TUM12-LexA-fw and TUM12-LexA-rv).
- The clean-up of the PCR product LexA (BBa_K105005) with primer containing the RFC25 pre- and suffix (TUM12-LexA-fw and TUM12-LexA-rv) was performed with the QIAquick PCR Purification Kit from Qiagen after manufacturer's protocoll.
- 5 µL of the purificated PCR product was taken to perform a analytical gelelectrophoresis to verify the success of the PCR.
- 1% agarose gel containing ethidium bromide was used for the analytical gelelectrophoresis.
- The analytical gelelectrophoresis was performed for 60 min at 90 V.
- Scheme of the gel:
| 100 bp ruler | PCR product of BBa_105005 | 1000 bp ruler |
LS: Plating of Schwab expression stains #106 & #108 which contain lavendula limonene synthase
Investigator: Lara Kuntz
Aim of the experiment: To get colonies of BL21 strains containing lavendula LS for amplification and subsequent plasmid extraction.
- Schwab strain #106 was plated on a chloramphenicol containing LB plate. #108 was plated on a amp+chlp containing LB plate. The plates were incubated at 37°C over night.
Quick Change mutagenis to remove PstI in the 2µ Ori from pYes2_RFC25 MCS
Investigator: Ingmar
Aim of the experiment: Removal of a PstI restriction site in the backbone of pYes2.
Operational sequence:
- From the transformation of the PCR-product of P35 two single clones were picked an transferred to 6 ml LB Amp. Incubation overnight at 37°C 180 rpm.
PCR of BBa_I742111 (Limonenesynthase) Clone 3 (Trafo 19.06.12)
Investigator: Andrea
PCR used forward primer with consensus sequence; PCR reaction mixture
| volume | reagent |
| 10 µl | 5x OneTaq Standard Reaction Buffer |
| 1 µl | 10 mM dNTPs |
| 1 µl | 10 µM Forward Primer O27 |
| 1 µl | 10 µM Reverse Primer O30 |
| 0.25 µl | OneTaq Hot Start DNA Polymerase (Finally: 1.25 units/50 µl) |
| 1 µl | Plasmid DNA (BBa_I742111) Clone 3 |
| 35.75 µl | dd water |
| 50 µL | TOTAL |
PCR used forward primer without consensus sequence; PCR reaction mixture
| volume | reagent |
| 10 µl | 5x OneTaq Standard Reaction Buffer |
| 1 µl | 10 mM dNTPs |
| 1 µl | 10 µM Forward Primer O28 |
| 1 µl | 10 µM Reverse Primer O30 |
| 0.25 µl | OneTaq Hot Start DNA Polymerase (Finally: 1.25 units/50 µl) |
| 1 µl | Plasmid DNA (BBa_I742111) Clone 3 |
| 35.75 µl | dd water |
| 50 µL | TOTAL |
- The PCR program was performed after following scheme:
| Initial denaturation | 94 °C | 30 s |
| 30 cycles | 94 °C | 30 s |
| 47 °C | 30 s | |
| 68 °C | 1,75 min | |
| Final extension | 68 °C | 5 min |
| Hold | 4 °C | 1 h |
Analytical Gelelectrophoresis
- 5 µl DNA + 1 µl loading buffer
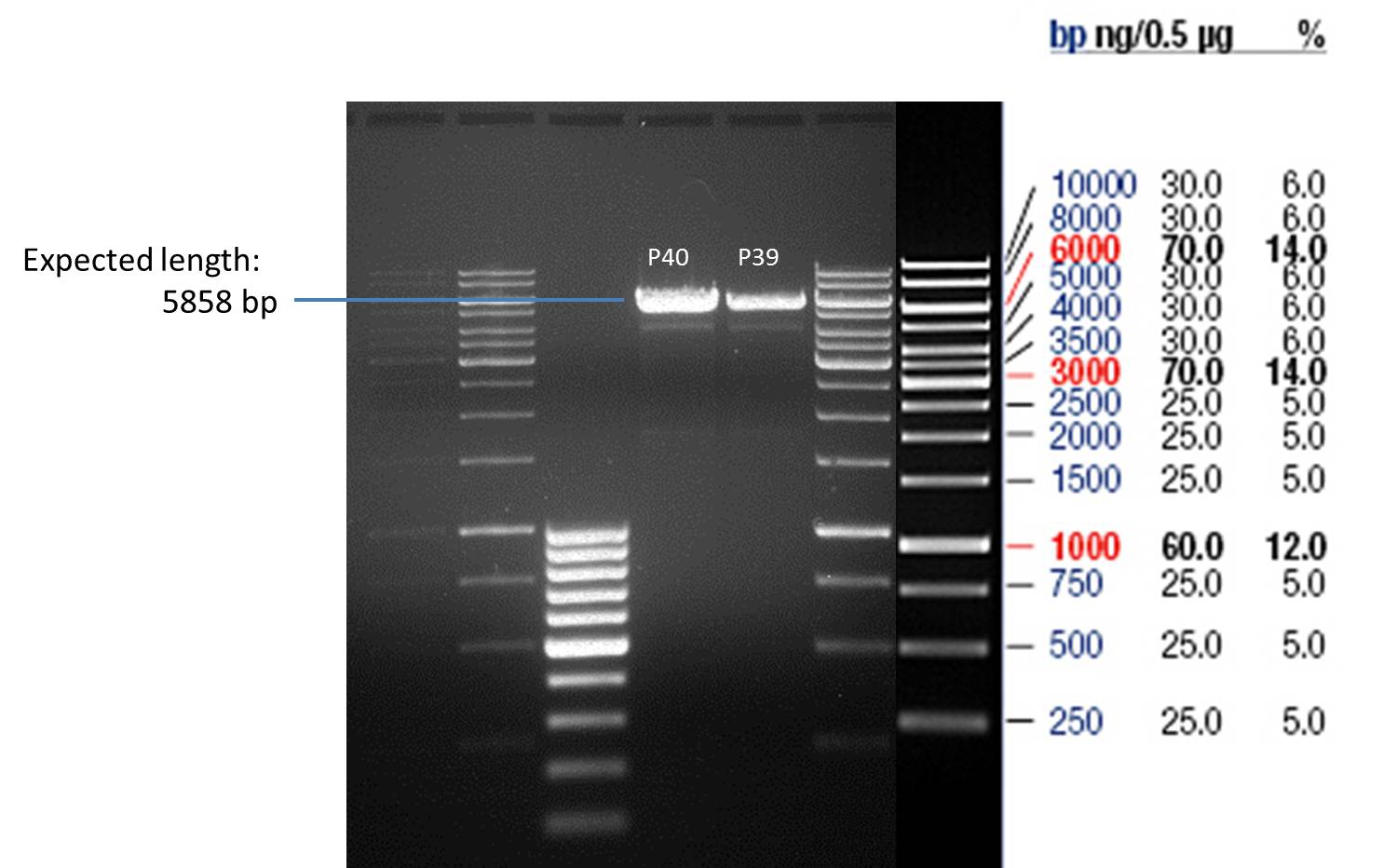
Wednesday, July 4th
Quick Change mutagenis to remove PstI in the 2µ Ori from pYes2_RFC25 MCS
Investigator: Ingmar
Aim of the experiment: Removal of a PstI restriction site in the backbone of pYes2.
Operational sequence:
- Using a Quiagen kit a miniprep of the overnight culture was done.
- The resulting purified DNA was aliquoted in new tubes labeled as follows:
1st Transformation of P35: P39
2nd Transformation of P35: P40
- Afterwards a control digestion of P39 and P40 was done.
Reaction batch
| Plasmid | P39 | P40 |
| Fermentas 10x R buffer | 0.5 µl | 0.5 µl |
| DNA | 5 µl | 5 µl |
| PstI | 0.25 µl | 0.25 µl |
| ddH2O | 14.25 µl | 14.25 µl |
| Sum | 20 µl | 20 µl |
- Incubation at 37 °C for 1h.
- Verification of control digest by agarose gel electrophoresis:
20 µl of each digest product was mixed with 4 µl 6x DNA loading buffer and loaded into the gel. The separation process lasted 1h at 90 V.
- All digest products show the expected bond at 5858 bp. The Miniprep product P40 was chosen to be used for the further Quickchange Mutagenesis.
Quick Change mutagenis to insert Ala in front of the Strep - tag II in pYES2_RFC25 MCS
Investigator: Ingmar
Aim of the experiment: Generation of an RFC 25 compatible version of the pYes2 Vector; operating purfication possibility via Strep-tag II.
PCR
Reaction batch
| volume | reagent |
| 2.5 µl | 10x Pfu Ultra II buffer |
| 4 µl | Plasmid P40 |
| 1 µl | 1:10 dilution of O54 (10 pmol/µL) |
| 1 µl | 1:10 dilution of O55 ((10 pmol/µL) |
| 16 µl | ddH2O |
| 0.5 µl | dNTP mix |
| 0.5 µl | Pfu Turbo DNA polymerase (2.5 U / µl) |
PCR cycling parameters
| Segment | Cycles | Temperature | Time |
| 1 | 1 | 95 °C | 30 sec |
| 2 | 16 | 95°C | 30 sec |
| 55°C | 1 min | ||
| 68°C | 6 min |
- Digestion of the parental DNA with DpnI: Addition of 1 µl DpnI to the PCR batch and incubate for 1 h at 37 °C.
Transformation into E.coli Xl1-Blue Operation Sequence
- melting of 100 µl Ca-competent E.coli XL1-Blue cells
- addition of 1 µl of the PCR product
- incubation for 30 min on ice
- heat shock for 5 min at 37 °C
- transfer of cells to 1 ml LB-medium without antibiotics and incubate at 37°C and 180 rpm for 30 min
- plate 100 µl on an Amp-LB-plate
- sediment the leftover in a centrifuge (30 - 60 sec, 13 000 rpm) and resuspend the sediment in 100 µl LB-medium and plate it as well on an Amp-LB-plate
Analytical Gelelektrophoresis of PCR-Products of PAL
Investigator: Mary
Aim of the experiment: purification and testing if PCR was successful
Purification of PCR-Products with purification kit from quiagen analytical gelelectrophoresis of PAL+, PAL-; expected band at 2,1 kb
piture follows
-> PCR was not successful
Schwab expression stains #106 & #108 which contain lavendula limonene synthase
Investigator: Andrea
Aim of the experiment: Getting clones of cells with plasmids containing the gene for limonenesynthase
Picking of Clones
- 2 clones of every stain were picked
- Incubation at 37 °C in LB + Amp (#108) / LB + Kan (#106)
Preparative Gelelectrophoresis of PCR-products of OMT
Investigator: Mary, Katrin
Aim of the experiment: Purification of the previously digested DNA, test if digestion was successful
picture follows!
Gelextraction of OMT- and OMT+ (bands are as expected; +=with consensus-sequence, -=without consensus-sequence)
DNA-purification with Kit from Quiagen
Thursday, July 5th
Preparation of plasmids containing lavendula LS
Investigator: Lara
Aim: Purify Schwab plasmids containing lavendula limonene synthase
Experiment was conducted using Qiagen Plasmid Miniprep Kit.
P39: Plasmid from Schwab strain #106 (1); 35 ng/µl
P40: Plasmid from Schwab strain #106 (2); 72 ng/µl
P41: Plasmid from Schwab strain #108 (1); 240 ng/µl
P42: Plasmid from Schwab strain #108 (2); 120 ng/µl
Restriction digest of Schwab plasmids
Investigator: Lara
Aim: To check whether extracted plasmids from Schwab expression strains #106 & #108 are OK.
Digest of plasmid from strain #106 was conducted with the following protocoll:
500 ng Plasmid DNA
0,25 µl Nco1
0,25 µl Hind 3
2 µl Buffer Tango
dd H2O to a total volume of 20 µl.
Digest of plasmid from strain #108 was conducted with the following protocoll:
500 ng Plasmid DNA
0,25 µl EcoR1
0,25 µl Not1
2 µl Buffer Orange
dd H2O to a total volume of 20 µl.
(All enzymes and buffers were from Fermentas).
Analytical gel electrophoresis of digested Schwab plasmids
Investigator: Lara Aim: Check plasmids for insert.
Plasmids were digested, 2 µl loading dye (10x) was added to each sample. Gel was loaded in the following order:
1. 6 µl gene ruler 1 kb, 2.-6. 11 µl of: P39, P40, P41, P42, Coumaryl-Plasmid (Kathrin)
Analytical Gelelektrophoresis of plasmid pKS2µHyg-PAL-4Cl-CHS
Investigator: Katrin
Aim of the experiment: testing if PAL is part of the plasmid that was sent to us (troubleshooting because PCR of PAL was not successful)
digestion took 1 h at 37°C
- digestion with ApaI (Fermentas)
| volume | reagent |
| 9.3 µl | plasmid pKS2µHyg-PAL-4Cl-CHS (miniprep) |
| 2 µl | Buffer B |
| 0.5 µl | ApaI (Fermentas; 10u/µl) |
| 8.2 µl | bidest. sterile H2O |
analytical gelelectrophoresis: expected band at 3,14 kb (Gal-PAL-XK)
picture follows
-> experiment was successful, PAL is part of the plasmid pKS2µHyg-PAL-4Cl-CHS
 "
"