Team:Peking/Modeling/Luminesensor/Orthogonality
From 2012.igem.org
Orthogonal Test in silico
To modularize the genetic system, our Luminesensor is expected to be bio-orthogonal with the origin system in bacteria. LexA, a natural element from the lactin-SOS system in bacteria may cause unexpected crosstalk. In order to remove this obstacle on the application prospects of our Luminesensor, we use LexA408 instead of the wild-type LexA. LexA408 and LexA are bio-orthogonal with each other since the sequence of the binding sites have variations.
By adding several nodes into the network, we constructed modeling for orthogonality test:
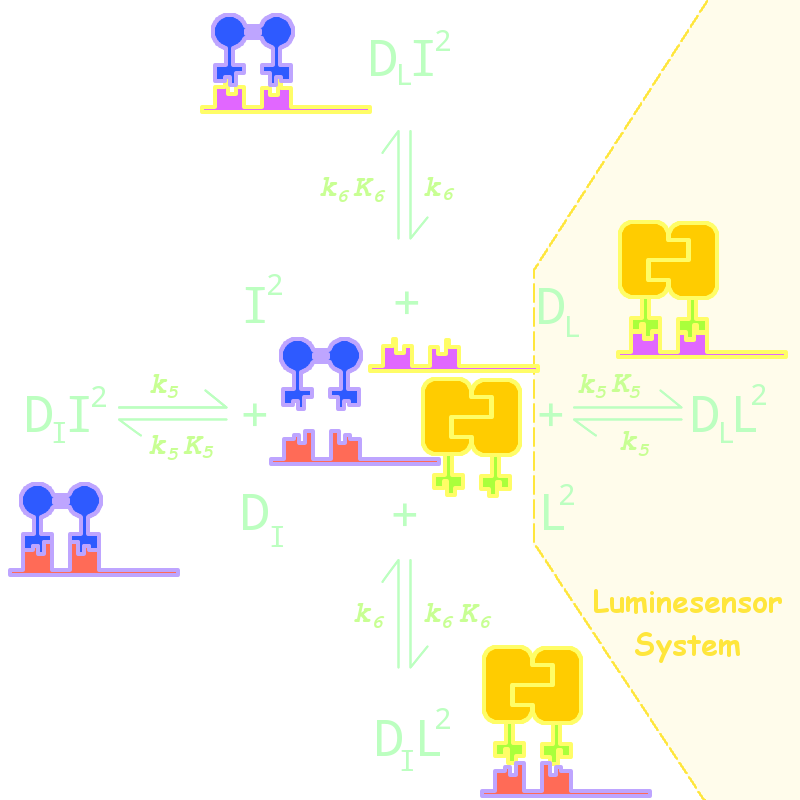
Fig 1. Kinetic Network for Orthogonal Analysis
where
- L denotes Luminesensor
- I denotes the inner wild LexA
- DL denotes the specific DNA binding site to Luminesensor
- DI denotes the specific DNA binding site to wild LexA
The parameters are estimated as following:
| Parameter | Value | Unit | Description |
| k6 | 1.x10-4 | s-1 | dimered LexA releasing rate constant from non-specific binding site |
| K6 | 1.x10-2 | (n mol/L)-1 | dimered non-specific binding equilibrium constant |
Tab 2. Reaction Parameters for Orthogonal Test
Fig 7. Orthogonal Test Result, the left show the simulation by ODE model and the right shows the stochastic simulation. (a) the origin system. (b) competition induced. (c) competition induced and tuning K6 up by 10 times.
The result shows that the endurance of K6/K5 is up to around 1%. Our Luminesensor can be used in bacteria with wild-type LexA.
Conclusion
The modeling above points out a way to optimize our Luminesensor -- the two critical mutation. It also shows the system still works well even if considering noise and inner competition.
Referrence
- [1] Light Activation of the LOV Protein Vivid Generates a Rapidly Exchanging Dimer. B. D. Zoltowski etc. Biochemistry
- [2] LexA Repressor Forms Stable Dimers in Solution. R. Mohana-Borges etc. THE JOURNAL OF BIOLOGICAL CHEMISTRY
- [3] Mechanism-based tuning of a LOV domain photoreceptor, Brian D. Zoltowski, etc. NATURE CHEMICAL BIOLOGY
- [4] Protein Vivid Generates a Rapidly Exchanging Dimer, Brian D. Zoltowski, etc. BIOCHEMISTRY
- [5] A new LexA-based genetic system for monitoring and analyzing protein heterodimerization in Escherichia coli, M. Dmitrova. etc. Springer-Verlag Mol Gen Genet
 "
"










