Team:Osaka/Project
From 2012.igem.org
| Line 46: | Line 46: | ||
電離放射線によるDNA損傷の種類は数多ある云々。 | 電離放射線によるDNA損傷の種類は数多ある云々。 | ||
Ionizing radiation causes atoms and molecules to become ionized or excited. These excitations and ionizations can | Ionizing radiation causes atoms and molecules to become ionized or excited. These excitations and ionizations can | ||
| - | + | produce free radicals, break chemical bonds, and produce new chemical bonds and cross-linkage between macromolecules. | |
| - | + | ||
| - | + | ||
*'''Mitomycin C''' | *'''Mitomycin C''' | ||
Revision as of 05:16, 26 September 2012
Project Details
iGEM Osaka 2012 project description
It is still sharp in our memory that, on March 11, 2011, the Great East Japan Earthquake struck off the coast of Eastern Japan and triggered a series of events that led to the nation wide nuclear crisis. Moved by that accident in iGEM 2011, we have built a synthetic biological dosimeter to detect the radiation. In this year we further develop that "Bio-dosimeter". Our "Bio-dosimeter"consists of two points: damage tolerance and radiation detection. To introduce the tolerance to E. coli, we are trying to put in some radiation resistance genes from Deinococcus radiodurans.For the detection of the radiation, we are trying to connect the native DNA damage response system of E. coli to production of pigment lycopene as a reporter. Now, we are attempting to assess its tolerance to various types of DNA damage and to evaluate DNA damage detection more clearly.
Damage tolerance
D. radiodurans
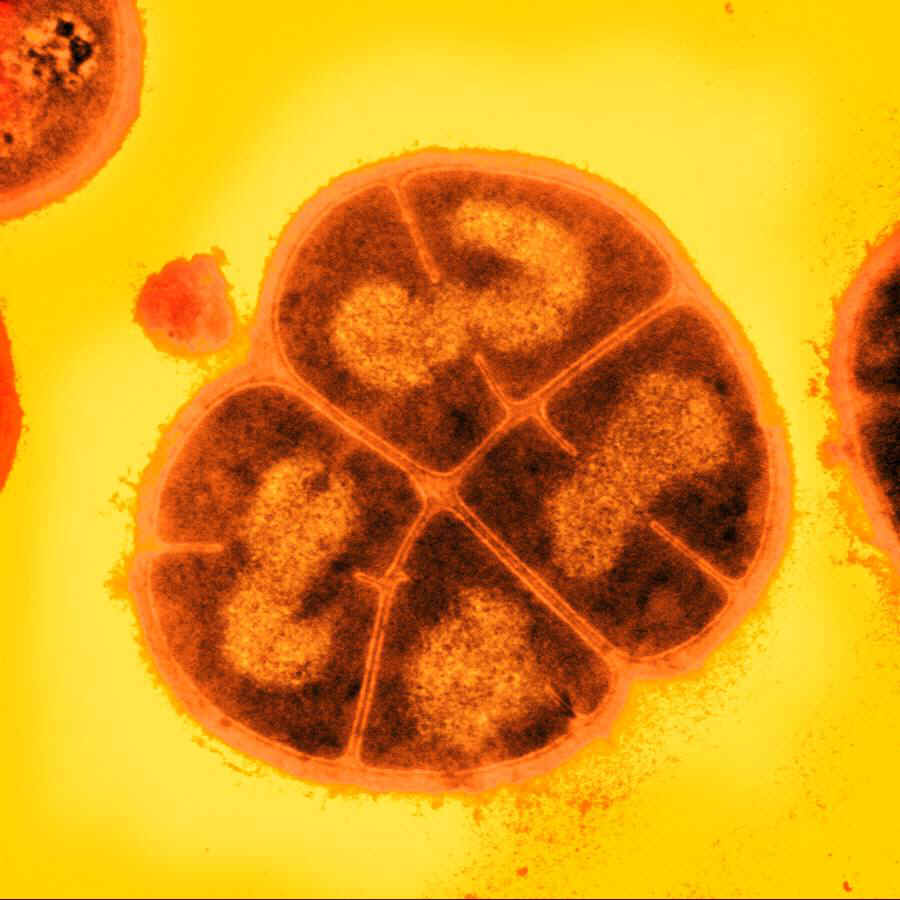
The bacterium Deinococcus radiodurans shows remarkable resistance to a range of DNA damage caused by ionizing radiation, desiccation, UV radiation, oxidizing agents, and electrophilic mutagens. It is an aerobically-growing bacterium that is most famous for its extreme resistance to ionizing radiation; it not only can survive acute exposures to gamma radiation that exceed 15,000 Gy, but it can also grow continuously in the presence of chronic radiation (60 Gy/hour) without any effect on its growth rate or ability to express cloned genes. For comparison, E. coli can withstand up to 200 Gy, and an acute exposure of just 5-10 Gy is lethal to a human being.
We explored various genes from D. radiodurans, implicated in its remarkable DNA damage resistance. By BioBricking selected genes and transforming them into E. coli, we hoped to confer additional DNA damage tolerance to the host cells.
Radiotolerance genes
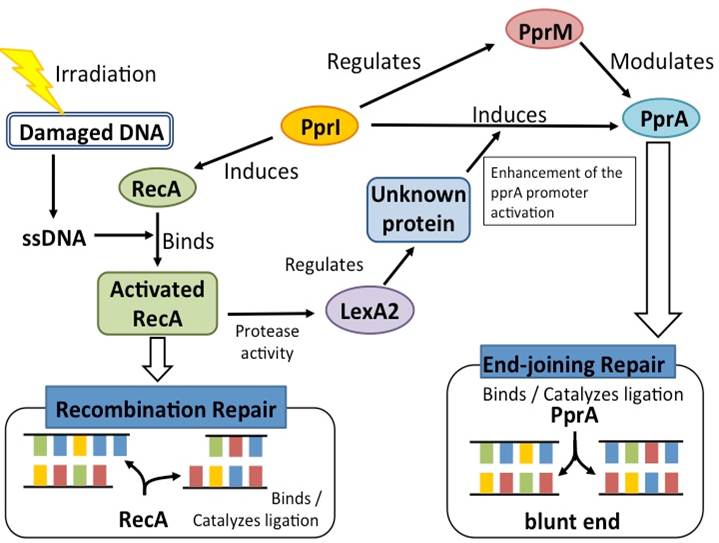
The D.radiodurans DNA repair system comprises many unique proteins.
- PprI
PprI, which is unique to D. radiodurans, is invoked by present data as the most important protein for radiation response mechanism. PprI can significantly and specifically induce the gene expression of recA and pprA and enhance the enzyme activities of catalases. These results strongly suggest that PprI plays a crucial role in regulating multiple DNA repair and protection pathways in response to radiation stress.
- PprA
A pleiotropic protein promoting DNA repair, its role in radiation resistance of Deinococcus radiodurans was demonstrated. PprA preferentially binds to double-stranded DNA carrying strand breaks, inhibits E. coli exonuclease III activity, and stimulates the DNA end-joining reaction catalysed by ATP-dependent and NAD-dependent DNA ligases. These results suggest that D. radiodurans has a radiationinduced non-homologous end-joining repair mechanism in which PprA plays a critical role.
- PprM
PprM (a modulator of the PprI-dependent DNA damage response) is a homolog of cold shock protein (Csp). PprM regulates the induction of PprA but not that of RecA. PprM belongs in a distinct clade of a subfamily together with Csp homologs from D. geothermalis and Thermus thermophilus. PprM plays an important role in the induction of PprA and is involved in the unique radiation response mechanism controlled by PprI in D. radiodurans.
- RecA
The D. radiodurans RecA protein has been characterized and its gene has been sequenced; it shows greater than 50% identity to the E. coli RecA protein. D. radiodurans recA mutants are highly sensitive to UV and ionizing radiation. In this context, early work by Carroll et al (1996) reported that E. coli RecA did not complement an IR-sensitive D. radiodurans recA point-mutant (rec30) and that expression of D. radiodurans RecA in E. coli was lethal. More recently, however, it has been reported that E. coli recA can provide partial complementation to a D. radiodurans recA null mutant (Schlesinger, 2007).
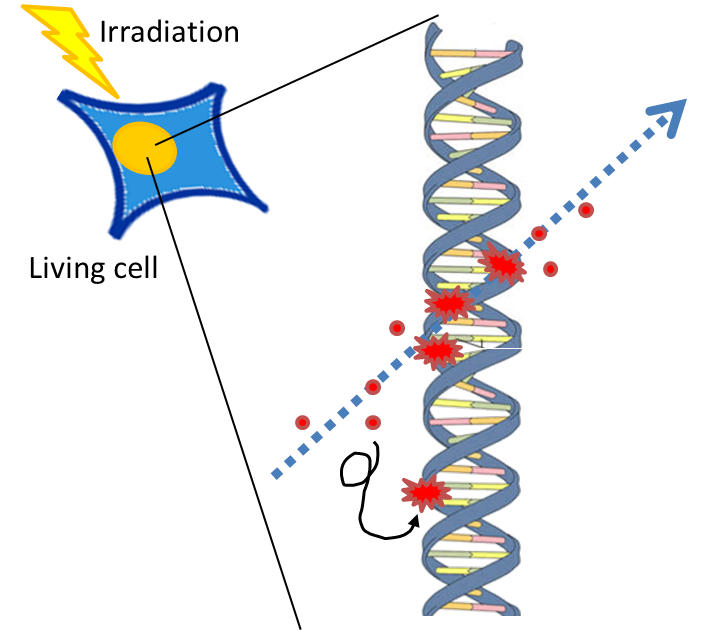
The effects of ionizing radiation
電離放射線によるDNA損傷の種類は数多ある云々。 Ionizing radiation causes atoms and molecules to become ionized or excited. These excitations and ionizations can produce free radicals, break chemical bonds, and produce new chemical bonds and cross-linkage between macromolecules.
- Mitomycin C
マイトマイシンCによるDNAの損傷について The DNA-damaging agent mitomycin C is known to introduce interstrand cross-links into duplex DNA. A single crosslink per genome has shown to be effective in killing bacteria. This is accomplished by reductive activation followed by two N-alkylations. Both alkylations are sequence specific for a guanine nucleoside in the sequence 5'-CpG-3'. Potential bis-alkylating heterocylic quinones were synthetised in order to explore their antitumoral activities by bioreductive alkylation. Mitomycin is also used as a chemetherapeutic agent in Glaucoma Surger. Mitomycin C is reduced in cells and bonds with the specific base sequence. When the bonding mitomycin C is oxidized automatically, ROS is produced and it breaks DNA chains near the bond site. Mitomycin C (MMC), a quinone-containing antitumor drug, has been shown to alkylate DNA and to form DNA cross-links. The ability of MMC to alkylate O6-guanine and to form interstrand cross-links (ISC) has been studied using Mer+ and Mer- human embryonic cells. Mer+ (IMR-90) cells have been reported to contain an O6-alkylguanine transferase enzyme and are, in general, more resistant to alkylating agents than the Mer- (VA-13) cell line, which is deficient in the repair of O6-lesions in DNA. Studies reported here show that MMC is more cytotoxic to VA-13 cells compared to IMR-90 cells. http://www.ncbi.nlm.nih.gov/pubmed/2550152
- Hydrogen peroxide
Hydrogen peroxide is known to highly reactive product. Therefore, when hydrogen peroxide is added to the cell, it breaks not only DNA molecules but also various substances in the cell, such as cell membrane, cell culture.(This is contrasting to the fact that MitomycinC specifically breaks DNA molecules.)
Radiation detection
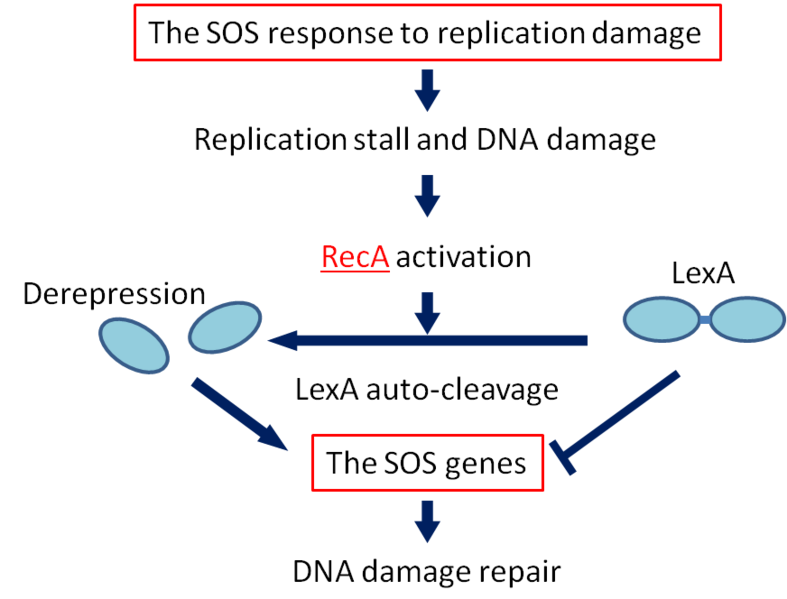
SOS response
If DNA is significantly damaged (eg by exposure to UV radiation or chemicals), synthesis of several DNA damage-related proteins occurs quickly. This reaction to DNA damage is the SOS response.
RecA is a 38 kilodalton Escherichia coli protein essential for the repair and maintenance of DNA. RecA has multiple activities, all related to DNA repair. In the bacterial SOS response, it has a co-protease function in the autocatalytic cleavage of the LexA repressor and the λ repressor. LexA is expressed constitutively and prevents expression of damage-related proteins by binding to SOS box as a repressor. RecA is activated by binding to single-stranded DNA, and the activated RecA then turns on LexA protease activity. Self-cleavage of LexA derepresses the expression of damage-related proteins enabling a response to be mounted.
We decided to employ the promoter of the RecA gene ([http://partsregistry.org/wiki/index.php?title=Part:BBa_J22106 BBa J22106]) to detect DNA damage. While RecA is an inducer of SOS genes, it itself is an SOS gene that is auto-induced upon DNA damage. Expression of genes downstream of this promoter is induced by DNA damage.
Lycopene biosynthesis
Our Bio-dosimeter must have some sort of visible output to alert users to radioactivity (detected as DNA damage). In a previous iGEM project, "colrcoli", we attempted to use E.coli as a paint tool. To that end, we examined biosynthesis of carotenoid pigments as a way of producing color. Here, we attempted to use biosynthesis of the carotenoid lycopene as a reporter for DNA damage.
Carotenoid is a family of natural pigments. Many plants such as fruits and vegetables contain these pigments. For example, tomato has lycopene(red), carrot has carotene(orange). Xanthophyll(yellow) is found in almost all plants.
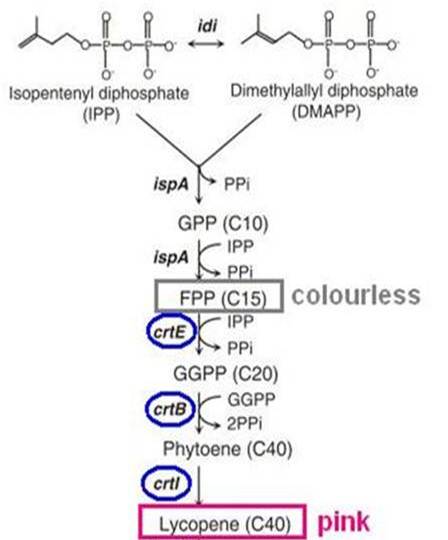
Biosynthesis of carotenoid pigments starts from FPP(FARNESYL DIPHOSPHATE). FPP is formed from isopentenylpyrophosphate(IPP) and dimethylallylpyrophosphate(DMAPP), which can be biologically produced by two distinct pathways, the mevalonate and non-mevalonate pathways. In E.coli, FPP is formed through the non-mevalonate pathway. By the introduction of heterologous enzymatic genes colorless FPP is then converted to orange-red lycopene, which has a peak absorbance at 407nm that is easily measured.
As shown in the figure on the right, the heterologous enzymes CrtE, CrtB, CrtI were introduced into E. coli to complete the biosynthesis of lycopene. We used a lycopene biosynthetic gene cluster provided by 2009 Cambridge (<partinfo>K274100</partinfo>).
References
- [1] Y. Hua et al., “PprI: a general switch responsible for extreme radioresistance of Deinococcus radiodurans,” Biochemical and Biophysical Research Communications, vol. 306, no. 2, pp. 354-360, Jun. 2003.
- G. Gao, B. Tian, L. Liu, D. Sheng, B. Shen, and Y. Hua, “Expression of Deinococcus radiodurans PprI enhances the radioresistance of Escherichia coli,” DNA Repair, vol. 2, no. 12, pp. 1419-1427, Dec. 2003.
- I. Narumi, K. Satoh, S. Cui, T. Funayama, S. Kitayama, and H. Watanabe, “PprA: a novel protein from Deinococcus radiodurans that stimulates DNA ligation,” Molecular Microbiology, vol. 54, no. 1, pp. 278-285, Oct. 2004.
- S. Kota and H. S. Misra, “PprA: A protein implicated in radioresistance of Deinococcus radiodurans stimulates catalase activity in Escherichia coli,” Applied Microbiology and Biotechnology, vol. 72, no. 4, pp. 790-796, Oct. 2006.
- H. Lu et al., “Deinococcus radiodurans PprI switches on DNA damage response and cellular survival networks after radiation damage,” Molecular & Cellular Proteomics: MCP, vol. 8, no. 3, pp. 481-494, Mar. 2009.
- H. Ohba, K. Satoh, H. Sghaier, T. Yanagisawa, and I. Narumi, “Identification of PprM: a modulator of the PprI-dependent DNA damage response in Deinococcus radiodurans,” Extremophiles: Life Under Extreme Conditions, vol. 13, no. 3, pp. 471-479, May 2009.
- Dusre L, Covey JM, Collins C, and Sinha BK, "DNA damage, cytotoxicity and free radical formation by mitomycin C in human cells." Chem Biol Interact. 1989;71(1):63-78
 "
"