Team:Evry/Protocols
From 2012.igem.org
Preparation of competent bacteria
A preculture of TOP10 cells grown in LB medium is provided
- Reseed 200ml of LB medium with 200µl of pre-culture. Incubate at 37°C (150rpm) until a DO600nm between 0,30 to 0,35 (exponential growth phase)
To do this, follow the evolution of DO600nm by making sterile samples of 1ml suspension. Take a first step after 1:30 of culture (generation time of bacteria is about 30min), then every half hour, using the same spectrophotometer for all measurements.
The cells must be kept on ice for the duration of the protocol
- Transfert the cells in four Falcon tubes previously cooled in ice.
- Incubate the culture 30min on ice.
- Centrifuge at 3000rpm for 5min at 4°C.
- Repeat all four base in 10ml of cold 0,1M CaCl2.
- Centrifuge at 3000rpm for 5min at 4°C.
- Resuspend the pellet in 10ml of 0,1M MgCl2 solution and incubate 30min on ice.
- Spin at 3000rpm for 5min at 4°C and add 2ml [CaCl2 + 10% glycerol].
- Aliquote cells in 1,5ml Eppendorf tubes previously cooled.
Store them on ice
Transformation
=> With chemically lab-made competent cells
- Keep constantly the cells on ice.
- Add 2-10µl of DNA ligation (2µl) or miniprep (2µl) in 100ul of competent bacteria (1µl of the positive control puc19).
- Incubate at least 30' on ice.
- Heat-shock the cells during 30 at 42°C in a water bath without shaking.
- Put cells for 2' on ice.
- Complete to 1 ml with pre-warmed SOC medium (42°C).
- Incubate 1h at 37°C under shaking (225 rpm).
- Take 100 µl and spread on appropriate plates.
- Incubate O/N at 37°C.
Midi-prep
Protocol from QIAGEN plasmid purification handbook
Pre-chill Buffer P3 at 4°C
1.Pick a single colony from a freshly streaked selective plate and inoculate a starter
culture of 2–5 ml LB medium containing the appropriate selective antibiotic. Incubate
for approx. 8 h at 37°C with vigorous shaking (approx. 300 rpm).
Use a tube or flask with a volume of at least 4 times the volume of the culture.
2.Dilute the starter culture 1/500 to 1/1000 into selective LB medium. For high-copy
plasmids, inoculate 25 ml medium with 25–50 μl of starter culture. For low-copy plasmids, inoculate 100 ml medium with 100–200 μl of starter culture. Grow at 37°C for 12–16 h with vigorous shaking (approx. 300 rpm).
Use a flask or vessel with a volume of at least 4 times the volume of the culture. The
culture should reach a cell density of approximately 3–4 x 109 cells per milliliter,
which typically corresponds to a pellet wet weight of approximately 3 g/liter
medium.
3. Harvest the bacterial cells by centrifugation at 6000 x g (6900rpm with rotor AV10/Ser N.0106/04/Cat N.11175754 - Centri CR31 Thermo) for 15 min at 4°C.
If you wish to stop the protocol and continue later, freeze the cell pellets at –20°C.
4. Resuspend the bacterial pellet in 4 ml Buffer P1.
For efficient lysis it is important to use a vessel that is large enough to allow complete
mixing of the lysis buffers. Ensure that RNase A has been added to Buffer P1.If LyseBlue reagent has been added to Buffer P1, vigorously shake the buffer bottle before use to ensure LyseBlue particles are completely resuspended. The bacteria should be resuspended completely by vortexing or pipetting up and down until no
cell clumps remain.
5. Add 4 ml Buffer P2, mix thoroughly by vigorously inverting the sealedtube 4–6 times, and incubate at room temperature (15–25°C) for 5 min.
Do not vortex, as this will result in shearing of genomic DNA. The lysate should
appear viscous. Do not allow the lysis reaction to proceed for more than 5 min. After
use, the bottle containing Buffer P2 should be closed immediately to avoid
acidification from CO2 in the air.
If LyseBlue has been added to Buffer P1 the cell suspension will turn blue after
addition of Buffer P2. Mixing should result in a homogeneously colored suspension.
If the suspension contains localized colorless regions or if brownish cell clumps are
still visible, continue mixing the solution until a homogeneously colored suspension
is achieved.
6. Add 4 ml of chilled Buffer P3, mix immediately and thoroughly by
vigorously inverting 4–6 times, and incubate on ice for 15 min.
Precipitation is enhanced by using chilled Buffer P3 and incubating on ice. After
addition of Buffer P3, a fluffy white material forms and the lysate becomes less
viscous. The precipitated material contains genomic DNA, proteins, cell debris, and
KDS. The lysate should be mixed thoroughly to ensure even potassium dodecyl sulfate
precipitation. If the mixture still appears viscous, more mixing is required to
completely neutralize the solution.
If LyseBlue reagent has been used, the suspension should be mixed until all trace of
blue has gone and the suspension is colorless. A homogeneous colorless suspension
indicates that the SDS has been effectively precipitated.
7. Centrifuge at ≥20,000 x g (10000rpm with rotor AV10/Ser N.0106/04/Cat N.11175754 - Centri CR31 Thermo)for 30 min at 4°C. Remove supernatant containing plasmid
DNA promptly.
Before loading the centrifuge, the sample should be mixed again. Centrifugation
should be performed in non-glass tubes (e.g., polypropylene). After centrifugation
the supernatant should be clear.
Note: Instead of centrifugation steps 7 and 8, the lysate can be efficiently cleared by
filtration using a QIAfilter Kits or Cartridges (see www.qiagen.com/products/
plasmid/LargeScaleKits).
8. Centrifuge the supernatant again at ≥20,000 x g (10000rpm with rotor AV10/Ser N.0106/04/Cat N.11175754 - Centri CR31 Thermo) for 15 min at 4°C. Remove
supernatant containing plasmid DNA promptly.
This second centrifugation step should be carried out to avoid applying suspended
or particulate material to the QIAGEN-tip. Suspended material (causing the sample
to appear turbid) can clog the QIAGEN-tip and reduce or eliminate gravity flow.
9. Equilibrate a QIAGEN-tip 100 by applying 4 ml Buffer QBT, and allow the column to empty by gravity flow.
Flow of buffer will begin automatically by reduction in surface tension due to the
presence of detergent in the equilibration buffer. Allow the QIAGEN-tip to drain
completely. QIAGEN-tips can be left unattended, since the flow of buffer will stop
when the meniscus reaches the upper frit in the column.
10. Apply the supernatant from step 8 to the QIAGEN-tip and allow it to enter the resin
by gravity flow.
The supernatant should be loaded onto the QIAGEN-tip promptly. If it is left too long
and becomes cloudy due to further precipitation of protein, it must be centrifuged
again or filtered before loading to prevent clogging of the QIAGEN-tip.
11. Wash the QIAGEN-tip with 2 x 10 ml Buffer QC.
Allow Buffer QC to move through the QIAGEN-tip by gravity flow. The first wash is
sufficient to remove all contaminants in the majority of plasmid DNA preparations.
The second wash is especially necessary when large culture volumes or bacterial
strains producing large amounts of carbohydrates are used.
12. Elute DNA with 5 ml Buffer QF.
Collect the eluate in a 15 ml or 50 ml tube (not supplied). Use of polycarbonate
centrifuge tubes is not recommended as polycarbonate is not resistant to the alcohol
used in subsequent steps.
13. Precipitate DNA by adding 3.5 ml (0.7 volume) room-temperature
isopropanol to the eluted DNA. Mix and centrifuge immediately at ≥15,000 x g for
30 min at 4°C. Carefully decant the supernatant.
All solutions should be at room temperature in order to minimize salt precipitation,
although centrifugation is carried out at 4°C to prevent overheating of the sample.
Alternatively, disposable conical bottom centrifuge tubes can be used for
centrifugation at 5000 x g (10000rpm with rotor AV10/Ser N.0106/04/Cat N.11175754 - Centri CR31 Thermo)for 60 min at 4°C. Isopropanol pellets have a glassy
appearance and may be more difficult to see than the fluffy, salt-containing pellets
that result from ethanol precipitation. Marking the outside of the tube before
centrifugation allows the pellet to be more easily located. Isopropanol pellets are also
more loosely attached to the side of the tube, and care should be taken when
removing the supernatant.
14. Wash DNA pellet with 2 ml of room-temperature 70% ethanol, and
centrifuge at ≥15,000 x g (10000rpm with rotor AV10/Ser N.0106/04/Cat N.11175754 - Centri CR31 Thermo)for 10 min. Carefully decant the supernatant without
disturbing the pellet.
The 70% ethanol removes precipitated
salt and replaces isopropanol with the more volatile ethanol, making the DNA easier
to redissolve.
15. Air-dry the pellet for 5–10 min, and redissolve the DNA in a suitable volume of buffer
(e.g., TE buffer, pH 8.0, or 10 mM Tris·Cl, pH 8.5).
Redissolve the DNA pellet by rinsing the walls to recover all the DNA, especially if
glass tubes have been used. Pipetting the DNA up and down to promote
resuspension may cause shearing and should be avoided. Overdrying the pellet will
make the DNA difficult to redissolve. DNA dissolves best under slightly alkaline
conditions; it does not easily dissolve in acidic buffers.
DNA purification phenol/chloroforme
Add 1 volume of phenol:chlorophorme:isoamyalcool (25:24:1)
Vortex
Centrifuge 10min, 14000rpm, 4°C
Recovery the aqueous phase (sup) and transfert it in a new tube
Add 1 volume pf chloroforme:isoamylalcool (24:1)
Vortex
Centrifuge 10min, 14000rpm, 4°C
Recovery the aqueous phase (sup) and transfert it in a new tube
Precipitate by adding 0,7 volume of isopropanol and 0,3 volume of aminium acetate 7,5M
Mix by inversion and stock at -80°C for 30min or at -20°C over night
Centrifuge 30min, 14000rpm, 4°C
Eliminate the supernatant and wash the pellet with 500ul EtOH 70%
Centrifuge 10min, 14000rpm, 4°C
Eliminate EtOH and dry for 10min at room temperature
Resuspend the pellet in TE buffer 1X
PCR with Phusion High-Fidelity DNA Polymerase
Tube preparation
Put items in this order:
| Component | 50µl reaction | Comments |
| H2O | 32 | |
| 5x Phusion HF Buffer | 10 | |
| 10mM dNTPs | 1 | |
| Primer FW | 2 | Primers have to be at 10µM |
| Primer RV | 2 | Primers have to be at 10µM |
| Template DNA | 1 | |
| DMSO (optional) | 1,5 | recommended for GC-rich amplicons < 20kb |
| Phusion DNA polymerase | 0,5 |
Cycling instructions
| Cycle step | Temperature | Time | Cycles |
| Initial denaturation | 98°C | 4min | 1 |
| Denaturation | 98°C | 20s | 30 |
| Annealing | Lower Tm of primers | 30s | |
| Extension | 72°C | 30S/kb | |
| Final extension | 72°C | 10min | 1 |
| 4°C | hold |
Preparation of LB medium and LB Agar:
=> LB Agar :
-18,5g LB Agar
-300ml H2O
=> LB medium : -6g LB broth -300ml de H2O
Autoclaved at 250°C
Gel extraction
1. Excise the DNA fragment from the agarose gel with a clean, sharp scalpel under UV light.
2. Weight the gel slice in a colorless tube. Add 3 volumes of Buffer QG to 1 volume of gel.
3. Incubate at 50°C for 10 min, until the gel slice has completely dissolved.
4. The color of the mixture have to be yellow, otherwise add 10 µl of 3M sodium acetate.
5. Ass 1 gel volume of isopropanol to the sample and mix.
6. Place a spin column in a provided 2 ml collection tube.
7. To bind DNA, apply the sample to the column and centrifugat for 1 min. Discard the flow-through and place the column back into the same tube.
8. To wash, add 0,75 ml of buffer PE to the column and centrifugate for 1 min.Discard the flow-through and place the column back into the same tube.
9. Centrifugate the column in a 2 ml Collection tube for 1 min 17,900xg (13,000 rpm).
10. Place the column into a clean 1,5 ml microcentrifuge tube.
11. To elute DNA, add 15 µl of water to the column and centrifugate for 1 min.
In vitro fertilization of Xenopus tropicalis
2 or 3 days before the IVF
Pre-injection of frogs (males et females)
100µl of hCG (0,1UI/µl, do a dilution by 10 of the commercial solution)
D day
Test the injector before beginning
Inject the 100µl hCG (1UI/µl) in the morning
Isolate the animals : a female by box
Prepare :
Timer
Methylcellulose (put at room temperature)
Boxes special for injections
Materiel of injection
Capillaires ad hoc with oil
Solutions to inject (add 1µl of blue or red of Nile)
Solution L15/FCS :
- Petri dishes (1/femelle, d=10 cm) lined of L15/FCS (500µl)
- 2 tubes 1,5mL with 250µl of L15/FCS (one for each testicle)
Pestles in plastic cleaned to grind the testicles
MMR 0,1X
MMR 0,05X
MS-222 0,2%
Euthanasia of the male
Put the male in a box with MS-222 0,2% and wait until he doesn't response for pinches. Take the male out and process the dissection to extract the two testicles.
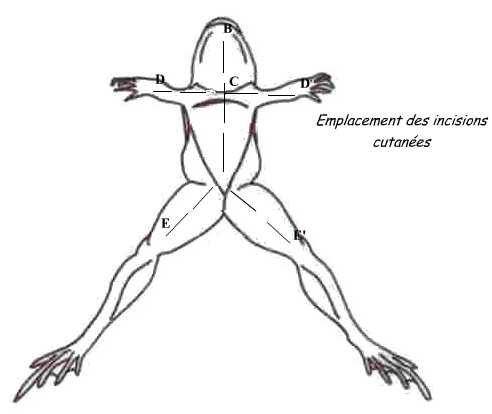
For that, dispose the animal: the dorsal face against the dissection board. Pinch the skin in the middle of the belly and do a small incision with scissors; from this point, perform skin incisions shown in the diagram.
Fold the flaps and skin incision in the muscular wall avoiding severing the abdominal vein to avoid hemorrhage. Both gonads are located in the ventral face of kidneys.
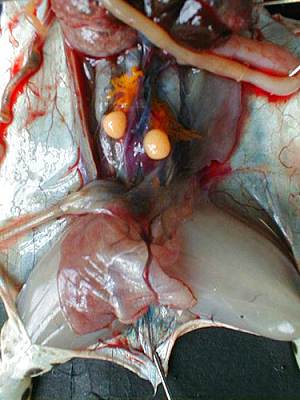
Both gonads are located in the ventral surface of the kidneys, they cover more or less and in corpora lutea (fat mass).By extracting them from the abdominal cavity, we can extract the testicles attached to them. The male's testicles are two oval organs, smooth, yellow-pale as can be seen in the photograph below.
Roll the testicles on kitchen paper to remove blood and blood vessels. Dip each testicle in a tube of 1,5mL with 250µl of L15/FCS. Keep tubes on ice. Crush the testicles with a pestle until obtaining a homogeneous solution. Add 250µl of L15/FCS, mix with a P1000 always on ice.
Lay eggs of females
Spawn females (who have already started laying alone) in Petri dishes previously coated with L15/FCS (gently massage into their flanks).
In vitro fertilization
With a pasteur pipette in plastic, remove as much liquid in the Petri dish prior the IVF. Add 50µl/500eggs of sperm solution on the eggs with P1000 and mix with a pipette tip to distribute the sperm on the eggs. Let the sperm fix on the eggs during 3-5 min.
Activate the spermatozoid by recovering the eggs of MMR 0,05X to allow the fecundation.
Dejellying embryos
15 min after fertilization activation, processing of the degangage.
For that, prepare cysteine 2%, pH 7,5-8 :
- Weight 2g of cysteine powder, suspend it in 80 mL of MMR 0,1% and put it in a beaker under magnetic agitation. Mesure pH and adjust it by adding NaOH 1M.
- Complete with MMR 0,1% to 100mL.
Remove MMR 0,05% from the Petri dish containing the eggs. Add the cysteine and agitate the plate by rotation for 2 min.
Transfer eggs to a 100ml pot (red plug) and continue to agitate by rotation for 5-8 min. Make sure that the embryos seem to touch each other to validate the degangage.
Rinse 3 times with MMR 0,1X.
Attention : during the diferent phases of washing, ensure that the eggs are always in liquid.
Add some methylcellulose to eggs to delay the developpement and facilitate the injection.
Place 50-100 eggs in a special injection Petri dish (lined with a small mesh) and inject DNA.
After injections, the embryos are transfered to MMR 0,1x in small petri dishes lined in the background with agarose. Boxes are incubated at 21°C.
Auxin toxicity test
Aim : test the toxicity of auxin
Preparation of stock solutions 0,05M
IAA (MW = 175 mol/g)
IAA powder stocked at -20°C
0,0876g in 10ml NaOH 0,1M
NAA (MW = 186 mol/g)
NAA powder stocked at room temperature
0,0931g in 10ml NaOH 0,1M
Stocked at 4°C
Day 1
Preparation of dilutions
Preparation of the mother solution
0,4ml IAA (or NAA) 0,05M
15ml MMR 0,1X
Ajust pH with HCl 1N
Fill up to the 20ml with MMR 0,1X
Solution n°1, 500uM :
7 ml mother solution + 7ml MMR 0,1X
Solution n°2, 250uM :
6ml solution n°1 + 6ml MMR 0,1X
Solution n°3, 125uM :
4ml solution n°2 + 4ml MMR 0,1X
Filling the wells with prepared solutions (~2ml/weel
| 12-wel plate | 1 | 2 | 3 | 4 |
| A | MMR 0,1X | Solution n°3 | Solution n°2 | Solution n°1 |
| B | MMR 0,1X | Solution n°3 | Solution n°2 | Solution n°1 |
| C | MMR 0,1X | Solution n°3 | Solution n°2 | Solution n°1 |
Add the embryos (see IVF protocol)
Add 10-30 embryos/well
Incubation
Incubator 21°C
Days after
Microscope observation
Take a picture of each well where it’s possible to see all the embryos
Count the alive and dead embryos
Remove the dead embryos
Medium changing
Protocol of DAY 1 for the preparation
Incline the plate and pipet the medium with a Pasteur pipette
Add MMR 0,1X in each well
Incline the plate and pipet the medium with a Pasteur pipette
Add the new medium following this map:
| 12-wel plate | 1 | 2 | 3 | 4 |
| A | MMR 0,1X | Solution n°3 | Solution n°2 | Solution n°1 |
| A | MMR 0,1X | Solution n°3 | Solution n°2 | Solution n°1 |
| A | MMR 0,1X | Solution n°3 | Solution n°2 | Solution n°1 |
Preparation of tadpole samples for HPLC
The original protocol from Vastag L, Jorgensen P, Peshkin L, Wei R, Rabinowitz JD, et al. (2011) Remodeling of the Metabolome during Early Frog Development. PLoS ONE 6(2): e16881. doi:10.1371/journal.pone.0016881 article was performed on 1 egg of Xenopus laevis. Because 1 embryo of this species represents 10 embryos of Xenopus tropicalis, we decided to perform our protocol on 10 Xenopus tropicalis embryos.
Aim: HPLC test to evaluate the presence of auxin in tadpoles embryos
Material
- IAA powder (stocked at -20°C)
- NAA powder (stocked at room temperature)
- Acetonitrile 100% HPLC grade
- Methanol 100% HPLC grade
- Falcon 15ml
- 1,5ml tubes
- Pasteur pipettes
- Mortar
- Centrifuge
- Ice
- Vortex
- P1000, P200 with tips
Prerequisite: To have perform a FIV and the auxin toxicity protocol the day before.
Before beginning: Turn on the centrifuge at 4°C
Preparation of solutions (under chemical hood)
Mix for 10 samples
2ml acetonitrile
2ml methanol
1ml H2O
Cool mix to -20oC
Prepare 1mg/ml auxin solutions in 20% acetonitrile/H2O
Preparation of the samples
Take 10 embryos from each concentration of IAA and NAA (0, 125, 250 and 500 µM)
Rinse the embryos 4 times in H2O
Place the embryos in 1,5ml tubes
Add 55µl of cold mix
Crush the embryos with a mortar
Vortex
Place on ice for 20min
Centrifuge 5min – 14000 rpm – 4°C (centrifuge eppendorf 5417R)
Collect as much as possible of the supernatant and place it in new tubes
To extract more:
Add 55µl of the mix on the pellet
Repeat all steps
Salkowski assay
Salkowski assay is the simplest way to test whether our samples (bacteria or tadpoles extracts)contain auxins.
Preparation of bacteria culture for assay
1. Measure the O.D. at 600nm of bacteria in order to observe difference in growth.
2. Centrifuge culture and filter supernatant through 0,2 µM filter.
Salkowski assay
1. Prepare the reagent:
Mix 0,5mL of FeCl3 0,5M with 25mL of HClO4 35%. Store in absence of light.
2. Add Salkowski reagent to the filtered supernatant in a ratio 2:1.
3. Incubate in dark for 30min.
4. Measure the D.O. at 530nm.
 "
"