Team:Shenzhen/Project/YAO.Suicider
From 2012.igem.org
-
S
BioBricks
- Summary
- YAO.Genome
- YAO.Channel
- YAO.Sensor
- YAO.Suicider
-
p
Notebook
- Team History
- YAO.Genome
- YAO.Channel
- YAO.Sensor
- YAO.Suicider
- YAO.Factory
-
e
Practices







- 1. Various Signaling?
- 2. Biosafer?
- 3. Signal imitation?
- 4. Holin kill eukaryotic?
- 5. Special signaling from mitochondria to nucleus?
- a. Signal Imitation
- b. Mitochondrion Dysfunction
- c. Retrograde Signaling from Mitochondrion
- d. Holin Production
- e. Holin Transformation and Destruction
- f. Apoptosis of Host
- For T7 Imitation System
- For Retrograde Signaling Part
- For Host Cell Suicide Part
Contents |
Introduction
YAO is like a BOMBER and cell is like whoever plays with fire and get burst. Once the fuse is ignited, YAO sacrifices, and following cascade reaction will lead to the cell repression!
More than crippling of YAO by DNase I, we creatively couple it with apoptosis of yeast cell!
Amazing Features:
This module ships signals from nucleus to mitochondria directly or reversely in four distinct ways, including T7 RNAP/T7 promoter coupling, peptide signaling, RTG pathway and cyt C-mediated apoptosis !
To guarantee biosafety, the engineered biofactory will die with its carrier cell !
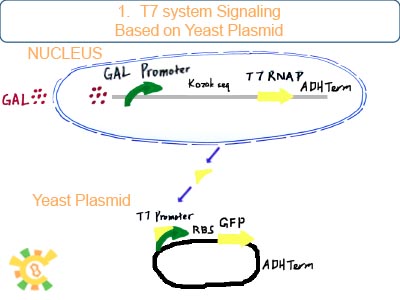
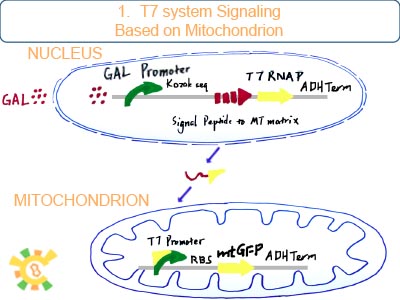
T7 RNAP/T7 promoter is employed to imitate the signal given to YAO instead of biosensor.
We try to illustrate that holin can punch not only the cytomembrane of bacteria but also the outer membrane of mitochondria-like YAO and ER to release cytC.
RTG pathway tell the cell that mt “Emergency~ Emergency~”, so cell supply it some replenishment.
Original Ideas
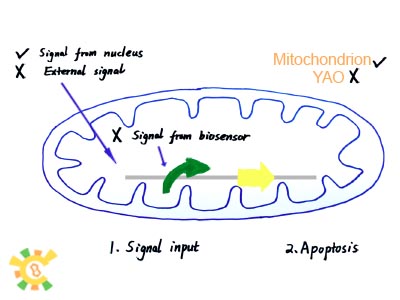
Originally, we try to construct such an artificial organelle YAO that YAO receives signal either from biosensor (internal) or from cytoplasm (external) or consequently trigger the host cell apoptosis. However, for YAO are not completed, we use mitochondrion as experiment target. And since we can’t rely on the availability of YAO.Sensor, the idea that Initial signal is given to YAO’s promoter is replaced by that nucleus produce T7 RNAP and transport it into mitochondrion to activate the T7 promoter inside it.
Final Story
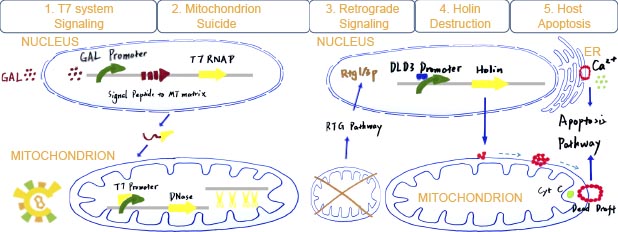
The final story is consist of six parts:
After galactose is added to the medium, RNAP can bind to Gal promoter and start transcribe and produce T7 RNAP with signal peptide to mitochondrial matrix. Then T7 RNAP gets into mitochondrial matrix.
Once T7 promoter works, DNase is produced to fragmentize the mtDNA which can lead to Respiration difficulty.
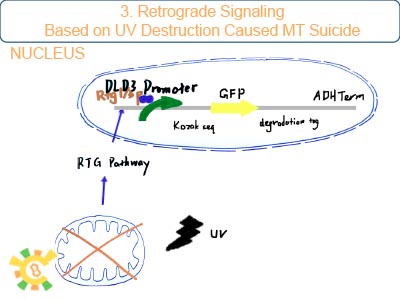
Mitochondrial dysfunction can trigger the RTG pathway which is the only known signaling from mt to nucleus.
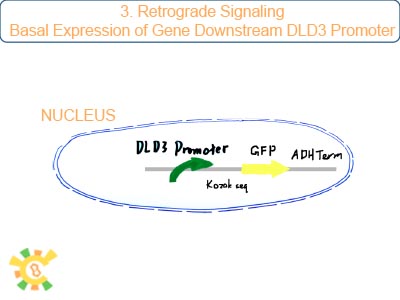
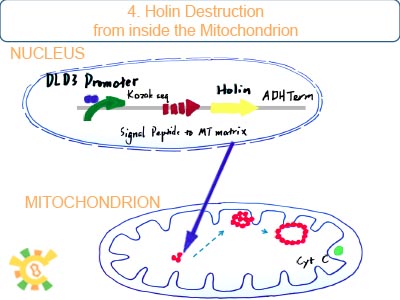
DLD3 promoter is the only stable downstream promoter relevant with RTG pathway. It can be binded and activated by RTG1/3p which is final production of RTG pathway. Once mitochondrial dysfunction exits, holin with DLD3promoter can be expressed.
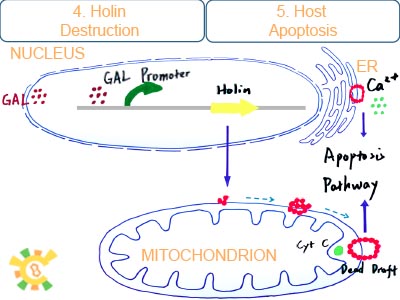
Once holin is produced, it can anchor on the outer membrane of mitochondria and ER, and form dimer with each other. Further more, they can form into a multidimer called dead draft if holin reachs a certain concentration. Dead drafts work like a large pore and release the apoptosis factor: cytochrome C, aif1 and calcium into cytoplasm.
Apoptosis factor cytochrome C and largely fleeing calcium can trigger the caspase-like pathway. Aif1 such as endo G can.get into nucleus and fragmentate DNA. Finally, host cell is suppressed or even die.
Verification Experiments Design
Because DLD3 is one of the target of RTG pathway of yeast, lowering the basal expression is required.
References
1. Prindle, A. et al. A sensing array of radically coupled genetic ‘biopixels’. Nature. 2011;481:39-44.
2. Liu, Z. Butow, R. A. Mitochondrial retrograde signaling. Annu Rev Genet. 2006;40:159-85.
3. Shi, Y. Sun, J.Current advance in the topological structure and function of holin encoded by bacteriophage lambda. Wei Sheng Wu Xue Bao. 2012 ;52:141-5.
4. Agu, C. A et al. Bacteriophage-encoded toxins: the lambda-holin protein causes caspase-independent non-apoptotic cell death of eukaryotic cells. Cell Microbiol. 2007:1753. Lipton, S. A.
5. Bossy-Wetzel, E. Dueling activities of AIF in cell death versus survival: DNA binding and redox activity. Cell. 2002;111:147-50.
6. Buttner, S. Endonuclease G regulates budding yeast life and death. Mol Cell. 2007 ;25:233-46.
7. Spencer, S. L. Sorger, P. K. Measuring and modeling apoptosis in single cells. Cell. 2011;144:926-39.
8. Li, P. Nijhawan, D. Wang, X. Mitochondrial activation of apoptosis. Cell. 2004;116:S57-9.
9. Pinkham, J. L. Dudley, A. M. Mason, T. L. T7 RNA polymerase-dependent expression of COXII in yeast mitochondria. Mol Cell Biol. 1994 ;14:4643-52.
 "
"