Team:TU Munich/Project/Thaumatin
From 2012.igem.org



Contents |
Thaumatin
Background and principles
Thaumatin is a natural α+β-protein which is synthesized by the katamfe plant (Thaumatococcus daniellii) – a species of tropical flowering plants- and belongs to the thaumatin-like protein family. There exist different varieties of thaumatin, however, thaumatin I und thaumatin II are well characterized and differ only in one position (Position 46 – without signaling sequence; thaumatin I Asn; thaumatin II Lys). Both are said to be 2000 to 100000 times sweeter than sucrose on molar basis, but the sweetness builds slow and lasts long.
Thaumatin is a single chain with 207 amino acids residues and eight disulfide bonds. It is highly water soluble, stable at heating (not for cooking, bakery, etc.) and stable under acidic conditions. The production of thaumatin is induced by an attack upon the plant by viroid pathogens. Thus it is involved in systematically acquired resistance and stress response.
Thaumatin has been approved as a sweetener in the European Union (E957).
The molecular and physiological effects of thaumatin:
The sweet taste receptor is a heterodimeric receptor composed of T1R2 (also TAS1R2) and T1R3 (also TAS1R3) subunits. The large amino-terminal domains (NTD) of the T1R2 and T1R3 subunits have shown to be responsible for the primary ligand binding (E. Maitrepierre et al. http://www.ncbi.nlm.nih.gov/pubmed?term=22450161). In addition these receptors have a transmembrane heptahelical domain. T1R receptors belong to the family of class C G-Protein coupled receptors (GPCRs), which in this case means that through ligand binding an elevation of the cAMP concentration in the taste buds is induced (N. Ide et al.http://www.ncbi.nlm.nih.gov/pubmed?term=19489607; M. Ozeck et al. http://www.ncbi.nlm.nih.gov/pubmed?term=15087236). As a result a decrease in the intracellular cAMP accumulation is measured. Released calcium (Ca2+) seems to be another independent second messenger within the transduction of the taste response (downstream of taste receptors) (KR. Trubey et al; http://www.ncbi.nlm.nih.gov/pubmed?term=16510847).
However, not only sucralose or other sugars can bind with the NTDs of the sweet taste receptor, but also thaumatin (N. Ide et al. http://www.ncbi.nlm.nih.gov/pubmed?term=19489607). It seems to have a longer lasting and stronger effect than sucralose.
Idea
The general idea is to create via genetic engineering of Saccharomyces cerevisiae a system that expresses thaumatin through a certain environmental stimulus. There are different options for an inducible promotor, however, after being induced the thaumatin gene respectively a precursor (preprothaumatin) should be expressed. One possible construct which could be used contains an ethanol inducible promotor (needs to be strong for the thaumatin expression), possibly an alpha-factor secretion signal plus the thaumatin I gene or the natural preprothaumatin I gene (for the secretion of the recombinant thaumatin) and some kind of resistance gene (e.g. blasticidin resistance). Preferable seems to be the natural preprothaumatin, because of the higher yield (page 377;http://www.jstage.jst.go.jp/article/jbb/102/5/375/_pdf) and the possibility that the pre-sequence is necessary for the correct procession (Ide et al.,Effects of pre- and pro-sequence of thaumatin on the secretion by Pichia pastoris; http://www.ncbi.nlm.nih.gov/pubmed?term=17897626). A similar construct was used by the Kyoto University (Ide et al., submitted) in Pichia pastoris with a the pPIC6α expression vector with a high yield (especially with the preprothaumatin I gene and without the α-factor secretion signal).
Remarks
Secretion
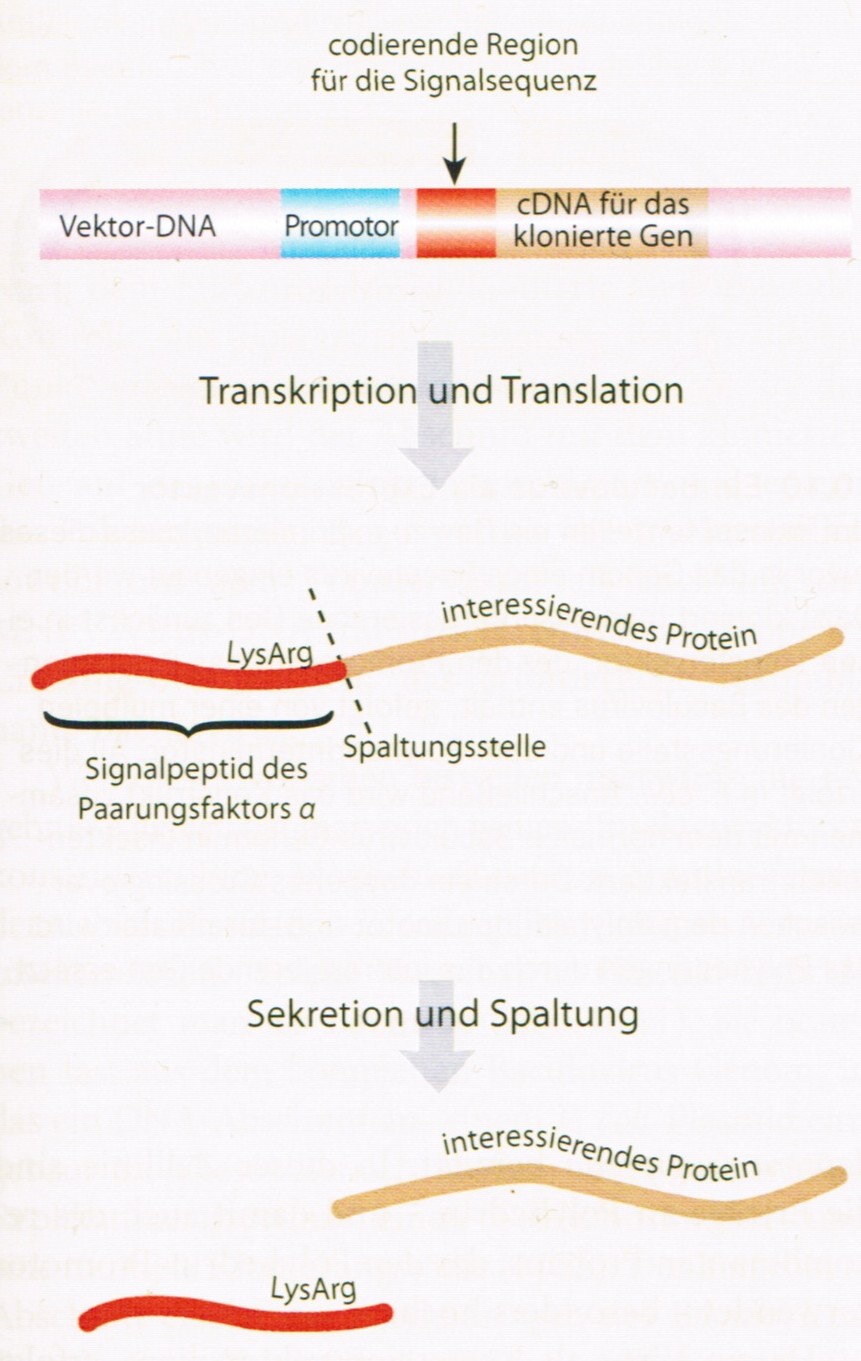
Recombinant proteins (in this case thaumatin) can be constructed for secretion by S. cerevisiae. The secretion is enabled by addition of the mating factor α upstream of the gene of interest. The signal peptidase of S. cerevisiae recognizes the sequences Lys-Arg after passing the membrane. This means the coding sequence needs to be directly downstream of the codons of Lys-Arg (David P. Clark, Nanette J. Pazdernik (2009); “Molekulare Biotechnologie”: 306 – 309).
The signal peptide of the mating factor α-1 (MF(ALPHA)1) begins with the first amino acid and ends with the 19th http://browse.yeastgenome.org/fgb2/gbrowse/scproteome/?name=YPL187W. Because of the fact that the signal peptidase of S. cerevisiae recognizes only the sequence Lys-Arg (KR) further amino acids until a KR-sequence appears need to be added. Another option is putting a genetically engineered KR-sequence after the signal peptide. It is probably safer to use the sequence until the first KR-sequence appears, because of the fact that afterwards (after the first KR-sequence) the actual mating factor alpha hormones are encoded. This option would mean to use the sequence until the 85th amino acid. http://browse.yeastgenome.org/fgb2/gbrowse/scproteome/?name=YPL187W As mentioned above (-> Idea) just using the preprothaumatin could also cause secretion. Different options should be considered! Through lab work the most effective alternative will be determined and then used in the next experiments.
Issues
Possible differences between P. pastoris and S. cerevisiae which might influence the production of recombinant thaumatin I should be researched. Codon-usage could become a problem. There are no thaumatin parts in the Registry of Standard Biological Parts. Because of the fact, that a “mating factor α-1 precursor secretion signal” http://www.biotechnologyforbiofuels.com/content/4/1/30 is needed upstream of the gene of interest, I am not sure whether this means just the signal peptide or a further part of the amino acid sequence. The MF(ALPHA)1 signal peptide is incompatible to the assembly standard 10 (has PstI).
Proof of principle
- First of all the existence of the preprothaumatin I / thaumatin I gene within the gene construct (an inducible promotor could be more useful for the proof) needs to be proofed (e.g. determination of DNA-length by gel electrophoresis and staining by EtBr; sequencing). In the next step gene expression needs to be measured (e. g. through gel electrophoresis and Northern blotting).
- Then protein secretion through mating factor α can be proofed by adding GFP or YFP gene downstream of the mating factor α gene. If there is fluorescence within the solution secretion can be proven.
- Afterwards the successful thaumatin gene can replace the fluorescence gene. Through SDS-PAGE and Western blotting thaumatin can be proven.
- If safety regulations permit the consumption of our product it can be proven by taste in the end.
References
Reviews (PMID)
- 3167035 http://www.ncbi.nlm.nih.gov/pubmed?term=3167035
- 17897626 http://www.ncbi.nlm.nih.gov/pubmed?term=17897626
- 14991654 http://www.ncbi.nlm.nih.gov/pubmed?term=14991654
- 10049878 http://www.ncbi.nlm.nih.gov/pubmed?term=10049878
- 21636903 http://www.ncbi.nlm.nih.gov/pubmed?term=21636903
- 19489607 http://www.ncbi.nlm.nih.gov/pubmed?term=19489607
- 22450161 http://www.ncbi.nlm.nih.gov/pubmed?term=22450161
- 15087236 http://www.ncbi.nlm.nih.gov/pubmed?term=15087236
- 16510847 http://www.ncbi.nlm.nih.gov/pubmed?term=16510847
Others
- Masuda and Kitabatake (2006), Development of Biotechnological Production of Sweet Proteins http://www.jstage.jst.go.jp/article/jbb/102/5/375/_pdf
- Thaumatococcus daniellii mRNA forpreprothaumatin I, comeplete cds http://www.ncbi.nlm.nih.gov/nuccore/121945717?report=genbank
- Preprothaumatin I [Thaumatococcus daniellii], FASTA http://www.ncbi.nlm.nih.gov/protein/121945718?report=fasta
- Masuda, Ohta, Mikami, Kitabatake (2011), High-resolution structure of the recombinant sweet-tasting protein thaumatin I http://repository.kulib.kyoto-u.ac.jp/dspace/bitstream/2433/142313/1/S174430911101373X.pdf
- Nobuyuki Ide, Tetsuya Masuda, Naofumi Kitabatake (2007), Effects of pre- and pro-sequence of thaumatin on the secretion by Pichia pastoris http://www.sciencedirect.com/science/article/pii/S0006291X07019900
- David P. Clark, Nanette J. Pazdernik (2009); “Molekulare Biotechnologie”: 306 – 309
- yeastgenome.org http://www.yeastgenome.org/
 "
"