Team:Tuebingen/Project
From 2012.igem.org
Contents |
Overview
Our general aim is to establish a simple synthetic organisim which will be capable to measure the influence of endocrine disruptors on the natural balance of sexual determination in all kind of vertebrates. The measurement itself will be cost-efficient, environment-friendly and sensitive.
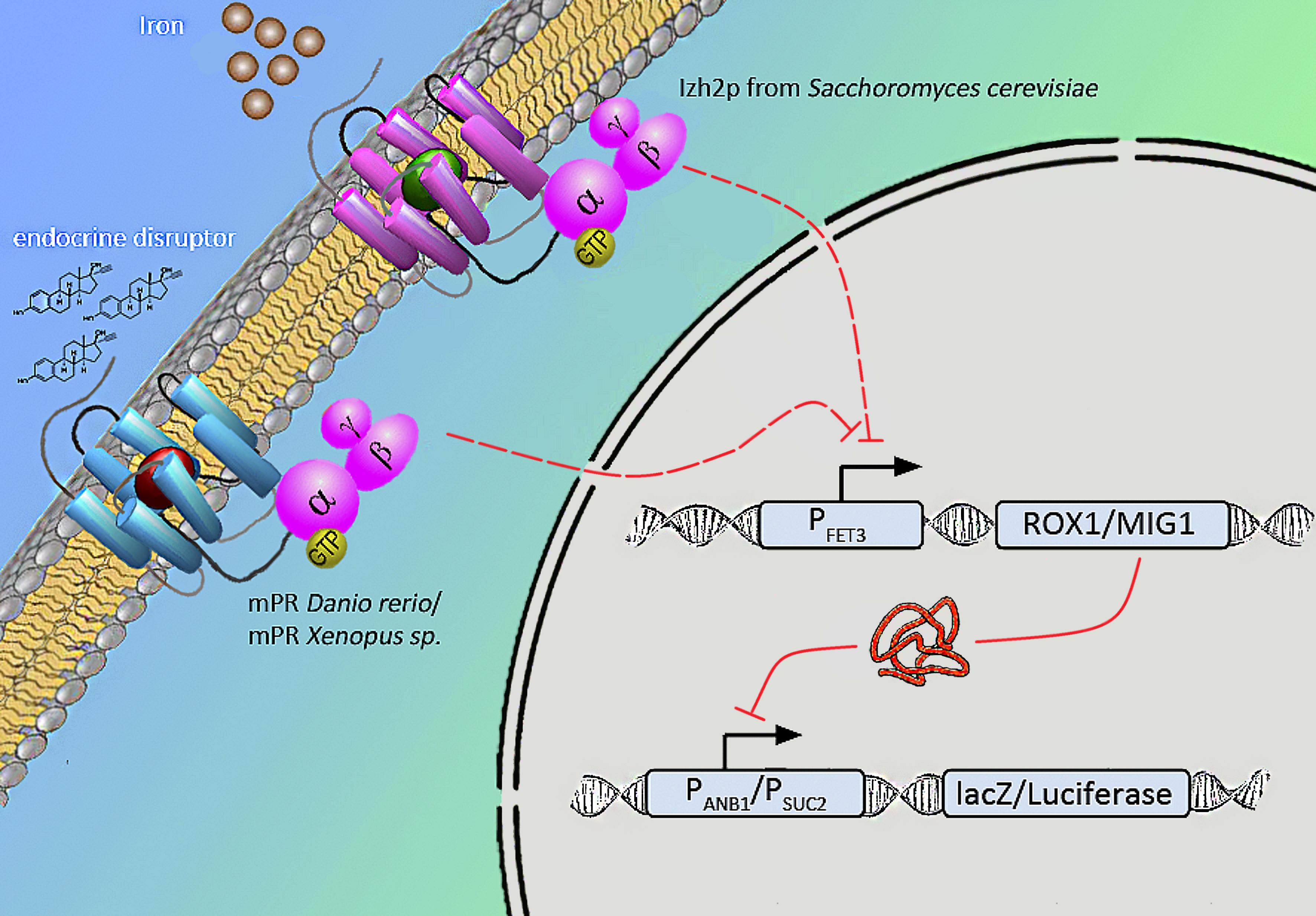
Naturally occuring iron receptors of the PAQR family are found to repress the fet3 promotor on high level of extra-cellular iron. According to Smith et al. human mPR expressed in yeast induced the same signal when binding to progesterone. Relying on this results we will express various mPRs of Danio rerio and Xenopus laevis in yeast to measure endocrine substances that influence fish and amphibians.
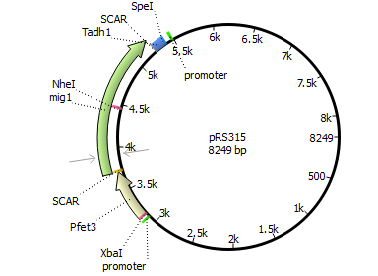
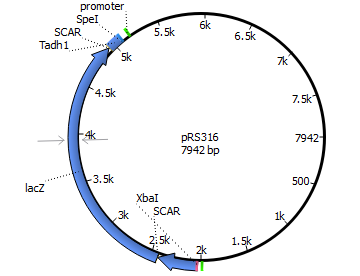
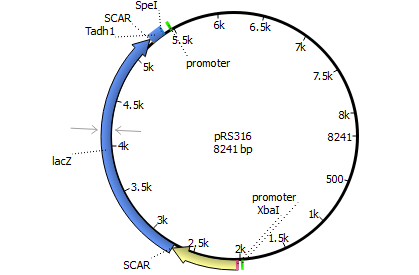
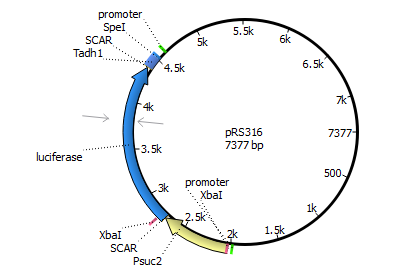
We will transform the negative signal (fet3 repression) into a positive signal by regulating a repressor (rox1 or mig1) with Pfet3. This repressor will in turn regulate the expression of the reporter gene (firefly luciferase or beta-galactosidase) and allow quantitative measurement.
Motivation
Why do we want to establish a mechanism for steroid measurement?
Steroid hormones, especially estrogens, occur in all vertebrates and play a crucial role in sexual differentiation. In recent times the pollution of waters with these hormones has become an increasing problem for the aquatic fauna.
Particularly waters functionalized by humans or adjacent to human settlements, e.g. in areas with agricultural use, show increased concentrations of estrogen.
Scientific studies based on Danio rerio showed that the consequences are devastating.
High concentrations of 17α-ethinylestradiol, a hormone in most birth-control pills, affected the sex differentiation of Danio rerio leading to development of ovotestis or complete feminization (Andersenc, 2002).
Intersex-fish have been reported in UK rivers since 1978 downstream of an sewage treatment plant.
We believe that a first step in finding a solution to this environmental problem is an accurate and reliable method to quantify steroid concentrations.
Occurring Questions
On our way designing the major pathway to express a specific reporter gene to demonstrate the presence of steroid hormones, we had and still have to deal with several questions concering the choice of BioBricks, genes and vectors to construct a firm method to determine "pollution" by steroids. As a conclusion, we have to meet two major requirements for our system:
- It should be as cost-efficient as possible for easy and regular application
- It should be resistant to yeast's own metabolism (Not be disturbed by unexpected occuring expression).
At first we had to find an appropriate receptor to "grab" steroid hormones in efluents. This should fulfill the following requirements:
- It should only be responsible to substrates we wish to detect, so the results of the test will not be falsified.
- It has to be easiliy integrated into yeast's cell membrane.
- Its nucleic acid sequence should not be too long so we can put it onto a plasmid vector.
We chose the membrane progesterone receptor of the zebra fish (Danio rerio) and the African clawed frog (Xenopus laevis). We focused on these receptors, since they are easy to duplicate and interact with a broad bandwith of sex-determining hormones.
The next step was to select an appropriate organism to express these receptors. After some research, we could narrow our options down to two organisms:
- Escherichia coli
- Saccharomyces cerevisiae
Finally we decided for yeast, since it has been done more research with it according to our prefered receptors. In addition yeast is an eukaryote making it more easier to integrate mPRs into their cell membrane.
Mechanism
Naturally occuring iron receptors of the PAQR family are found to repress fet3 promotor on high levesl of extra-cellular iron. According to [http://www.ncbi.nlm.nih.gov/pubmed/18603275 J Smith et al.] human mPR expressed in yeast induced the same signal binding to estrogen. Relying on this results we will try to express various mPRs of Danio rerio, Xanophus laevis in yeast which we find fitting to measure endocrine substances that influence fish.
We will transform the negative signal (fet3 repression) into a positive signal by regulating a repressor (rox1 or mig1) with Pfet3. This repressor will in turn regulate the expression of the reporter gene (firefly luciferase or beta-galactosidase) and allow quantitative measurement.
Receptors
Membrane progesterone receptors are membrane bound, seven-transmembrane receptors and belong to the PAQR family (progesterone adiponectin Q receptor). These G-protein coupled receptors activate inhibitory Gi units.
Inhibitors and their targets
An appropriate inhibitor/promotor combination is a crucial step in our pathway and should be selected wisely.
Finally we chose FET3 as our promoter and both ROX1 and MIG1 as inhibitors. It is very likely, that they don't seem to repress any gene expression that are crucial for yeast.
Reporter genes
The last station of our signaling pathway should be a reporter gene which amplifies our initial signal to allow a quantitative measurement.
The enzyme luciferase fulfills these conditions and is our candidate of choice.
Implementation
Promoter
Inhibitor
Reporter
Measurement
The measurement itself should be limited to an optical one. With this idea in mind, we decided for reporter genes like lacZ and luciferase, because these produce signals which can simply be quantified by optical measurement methods.
References
- [1] Jessica L. Smith, Brian R. Kupchak, Ibon Garitaonandia, L. Kim Hoang, Andrew S. Maina, Lisa M. Regalla, and Thomas J. Lyons - 2008 - Heterologous expression of human mPRα, mPRβ and mPRγ in yeast confirms their ability to function as membrane progesterone receptors
- [2] Sumpter, Johnson (2008) Reflections on endocrine disruption in the aquatic environment
 "
"