Team:Cambridge/Lab book/Week 7
From 2012.igem.org
| Week: | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|
Contents |
Monday (06/08/12)
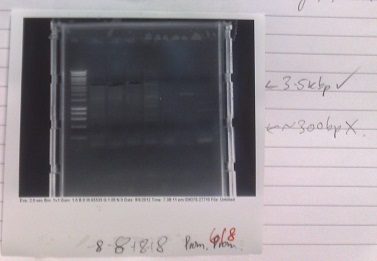
Ribosense & Existing Biobricks: PCR of Magnesium riboswitch vector fragment B and Magnesium promoter
- Normal PCR settings used, annealing temperature 57 °C, elongation step 90s long.
- Lane 5 accidentally loaded with a DNA ladder instead of loading dye.
- Expected fragment sizes:
- Lane 2-5: 3kbp
- Lane 6-7: 300bp
- After electrophoresis, found vector products had, for the most part, worked. Promoter elements were not amplified - no band of the expected size was observed.
- Positive control also failed, although this has ceased to work for several days, potentially due to DNA degradation.
Ratiometrica-Lux: Split PCR of Lux vector
- Primers for spliting pSB1C3 arrived and were used to PCR the Ratiometrica-Lux vector
- Fragment A: Vec FWD and Vec split RVS (expected size: 5kb)
- Fragment b: Vec split FWD and Vec RVS (expected size: 4.6kb)
- PCR Programme:
- Results: other than one very ambiguous band (lane 6) which might be of the right size, the others did not work.
Tuesday (07/08/12)
Production of electrocompetent e.coli
Ribosense: Gibson assembly of magnesium riboswitch
- NAD+ added to isothermal buffer*5 mix
- Gel slices from yesterday (of vector fragment B) purified.
- DNA added as follows:
- Without 8 codon substitution:
- Reaction 1: Tube 2 (07/08/12) (fragment B), Tube 1 (05/08/12) (fragment A), Tube 28 (29/08/12) (riboswitch DNA).
- Reaction 2: Tube 3 (07/08/12) (fragment B), Tube 2 (05/08/12) (fragment A), Tube 29 (29/08/12) (riboswitch DNA).
- With 8 codon substitution:
- Reaction 3: Tube 4 (07/08/12) (fragment B), Tube 1 (05/08/12) (fragment A), Tube 2 (18/07/12) (riboswitch DNA).
Ratiometrica-Flu: Gibson assembly of Fluorescent construct
- 6-piece Gibson assembly done in triplicate
- Reaction 1: Tube 1 (Fragment A), Tube 4 (Fragment B), Tube 10 (CFP), Tube 13 (B0015), Tube 16 (K143053), Tube 19 (YFP)
- Reaction 2: Tube 2 (Fragment A), Tube 5 (Fragment B), Tube 11 (CFP), Tube 14 (B0015), Tube 17 (K143053), Tube 20 (YFP)
- Reaction 3: Tube 3 (Fragment A), Tube 6 (Fragment B), Tube 12 (CFP), Tube 15 (B0015), Tube 18 (K143053), Tube 21 (YFP)
- TOP10 E.coli is transformed and plated onto 100μg/ml Ampicilllin plates
Wednesday (08/08/12)
Electrical transformation of competent e.coli with Gibson products
- Gibson constructs from yesterday (fluorescent and magnesium riboswitch) transformed into e.coli produced yesterday.
- Cells plated out onto 50 μg/ml ampicillin plates. Put in incubator overnight.
Thursday (09/08/12)
Making chemically competent e.coli
- 1l SOB made up.
- Split mOrange vector amplification attempted again.
Friday (10/08/12)
Characterization of fluoride riboswitch sensitivity with β-galactosidase
- X-Gal made up to a concentration of 400 μg/ml with water.
- Fluoride of concentrations add concentrations mixed with bacterial culture (Yale construct containing) grown up overnight.
Saturday (11/08/12)
File:Yalerestdig.jpg
The gel from our restriction digest. Different lanes represent different colonies that grew on our initial plates. The presence of bands at ... indicate that the correct plasmid DNA has integrated.
Restriction digest of Yale plasmid
- Cells from electroporation of e.coli with plasmid from Yale (08/08/12) miniprepped to extract plasmid DNA.
- DNA digested with What enzymes did you use, Jolyon?
- Gel run, gave results shown.
- Bands in correct places, indicating that expected plasmid was present in the cells. This indicates that, although they work at a very low efficiency, our electro-competent cells still work. It may be valuable to use these when trying to grow up plasmids that we already have at high concentration.
Transformation of e.coli with Magnesium riboswitch construct
- Gibson products from 07/08/12 transformed into chemically competent cells.
- Transformants plated out on 100 μg/ml ampicillin plates
Sunday (12/08/12)
Assembly of sfGFP construct from known functional DNA fragments
- Fragments provided by Fernan assembled with Gibson.
Transformation of e.coli with fluorescent construct and sfGFP positive control
- Chemically competent e.coli cells transformed with fluorescent construct Gibson product from 07/08/12.
- E.coli cells also transformed with sfGFP Gibson product made earlier today in triplicate.
- All transformants plated out on 100 μg/ml ampicillin.
- sfGFP fragments have produced successful Gibson products in the past, so this will act as our positive control. If cells grow with the plasmid and fluoresce properly, we will know the master mix works. Otherwise, we will remake the master mix.
 "
"