Contents |
Minipreps for pGL and pcDNA3.1(+)-LovTAP-VP16 C5 and C6
Protocol: Miniprep
The slim tubes can be centrifuged in the machine in front of the "Gel hood", at 4000 rpm for 10 min. The fatter ones, in the E. coli centrifuge by the fridge (the tip can be left inside, since it floats).
Pellets resuspended with RNase containing buffer (Resuspension Buffer R3, from Invitrogen, equivalent to Buffer P1 from Qiagen, in Sowmya's box in the fridge). Note: keep the buffer in ice if you are not bringing it back to the fridge for some minutes.
We then use the QIAGEN QIAprep Spin Miniprep Kit with their [http://www.qiagen.com/literature/render.aspx?id=370 protocol] (page 22) and a microcentrifuge.
Biobrick gradient PCR
Protocol: PCR
PCR is a reaction that makes it possible (and relatively easy) to amplify
a certain region of DNA. The first step is the selection of that region
(and the design of the relevant primers). Primer design can be done by hand, or by
using our Primer Design Helper. Once
done, order the primers (in our case, we ordered from them [http://www.idtdna.com/ IDT]).
When you've received the primers, prepare them and make sure you've got your PCR kit (we used the "Phusion® High-Fidelity DNA Polymerase"). Start preparing your master mix, the composition for one tube is:
1X Mastermix 20μl reaction, add in this order
| Reagent | Volume [μl] |
|---|---|
| Water | Complete to total volume of 20μl |
| HF-Buffer (5x) | 4 |
| DMSO (optional) | 0.6 |
| dNTPs | 0.4 |
| Forward primer (50μM) | 0.2 |
| Reverse primer (50μM) | 0.2 |
| Template (10ng/μl) | 0.5 |
| Phusion HF polymerase | 0.2 |
Prepare one or two extra tubes-worth of reagent (you'll use some liquid on the walls of your tips).
Once you've finished, you should run the resulting products on a gel to check if everything went as planned.
Tips
- Thaw the HF-Buffer, DMSO and dNTPs before making the mastermix.
- Avoid taking the Phusion-HF polymerase out of the freezer (only take it out briefly when you need to add it).
- If the reactions have different primers and/or template, add the polymerase right after the dNTPs, split the mastermix and add the rest.
- Don't forget positive and negative controls
- Primers should have similar Tms (less than 5°C).
- Primer Tm calculation is a less exact science than it should be (just test several tools and compare their results). If you're not sure what the correct Tm is, consider using a gradient PCR.
- Avoid primers with strong secondary structures.
- PCR can introduce mutations. Don't forget to sequence your final product (this could be your final plasmid): you really don't want to lose a few weeks because of a "corrupt" plasmid.
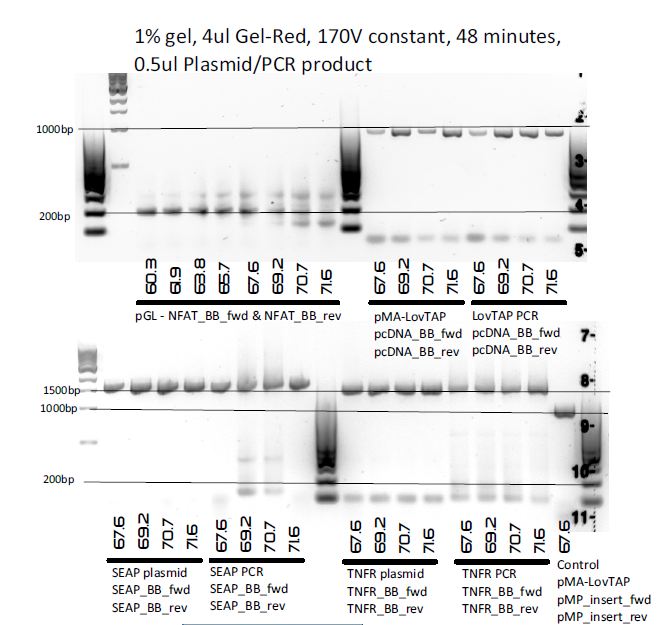
PCR to amplify as much inserts as possible for Biobricking.
Templates:
- pGL4.30 (for NFAT)
- pMP-PB-TNFR-Fc
- pMP-PB-SEAP-FLAG
- pMA-LovTAP
Eight 20μl reactions per Biobrick insert were made, so in total 32 main reactions and 1 control reaction (pMA-LovTAP with LovTAP_fwd and LovTAP_rev).
Results
pGL backbone plasmid digestion
With HindIII and MfeI, for ligation with SEAP.
Gel for digested pGL
More Amp plates prepared
Protocol: Agar Plates
- Add to a bottle:
- 20 g/l of LB broth powder.
- 10 g/l of Agar (not agarose).
- Fill up with DI water and autoclave (program 106, takes 2 hours). Remember to leave the cap loose!
- Label the plates (found in the stock room).
- When the autoclaving is done, close the bottle cap and take the bottle out.
- Let rest until it cools down to around 55ºC (can be held for some seconds): if warmer, the antibiotic degrades, if colder, the broth gelifies.
- Add the antibiotic (Ampicillin 100 µg/ml) = 1 ml of 100 mg/ml Amp for 1l of broth. Same for chloramphenicol or Spe.
- Add 25-30 ml of broth to each plate and let open for 2 hours.
- Close plates, wrap in alu foil (Amp is sensitive to light) and store at +4ºC.
 "
"