Contents |
Minipreps for pGL and pcDNA3.1(+)-LovTAP-VP16 C4 and C5
Protocol: Miniprep
The slim tubes can be centrifuged in the machine in front of the "Gel hood", at 4000 rpm for 10 min. The fatter ones, in the E. coli centrifuge by the fridge (the tip can be left inside, since it floats).
Pellets resuspended with RNase containing buffer (Resuspension Buffer R3, from Invitrogen, equivalent to Buffer P1 from Qiagen, in Sowmya's box in the fridge). Note: keep the buffer in ice if you are not bringing it back to the fridge for some minutes.
We then use the QIAGEN QIAprep Spin Miniprep Kit with their protocol (page 22) and a microcentrifuge.
Minipreps were made from the pGL4.30 cultures from the night before. We also made a miniprep of the C6 colony for sequencing.
Sequencing
Protocol: DNA Sequencing Sample Preparation
Microsynth
Microsynth is a Swiss sequencing company that has a pick-up service at EPFL, which means the sample doesn't need to be sent anywhere. It also has a library of standard primers, in case there is some kind of common sequence in the vicinity of the sequence of interest that could be used as the starting point for Sanger sequencing.
Requirements
- Design the sequencing primers that should start approximately 50 bp upstream and downstream of your sequence of interest. If there is a standard sequence in that area, pick a pre-made primer from the standard list. You can also order the primers from some other company and send them along with the DNA sample.
- Test your primers by running a virtual PCR with Serial Cloner or any other software on your sequence. If you get the expected product, they are correct. Log in to the Microsynth website, place your order and print out the primer barcode.
- Prepare the DNA of interest according to Microsynth guidelines. The plasmid concentration should be 80 ng/µl, and the total volume shall be 15 µl. Therefore, you need to take 1.2 µg of DNA from your original sample, and complete it to 15 µl with either pure water, either 10 mM Tris-HCl (pH 8), either 10 mM Tris-HCl (pH 8) with a maximum of 0.01 mM EDTA, depending on your needs. Use the tubes that are recommended by the company (screwcaps). Make two identical 15 µl samples for every DNA sample you want to sequence, one will be used for the forward primer, and one for the reverse one. Put prepaid Microsynth stickers on them.
- Leave the DNA sample and the primer barcode in the EPFL pick-up area (SV building, level 0). They will be shipped off to Microsynth.
The sequencing sample of C6 was shipped off to Microsynth.
Biobrick gradient PCR
Protocol: PCR
PCR is a reaction that makes it possible (and relatively easy) to amplify
a certain region of DNA. The first step is the selection of that region
(and the design of the relevant primers). Primer design can be done by hand, or by
using our Primer Design Helper. Once
done, order the primers (in our case, we ordered from them IDT).
When you've received the primers, prepare them and make sure you've got your PCR kit (we used the "Phusion® High-Fidelity DNA Polymerase"). Start preparing your master mix, the composition for one tube is:
1X Mastermix 20μl reaction, add in this order
| Reagent | Volume [μl] |
|---|---|
| Water | Complete to total volume of 20μl |
| HF-Buffer (5x) | 4 |
| DMSO (optional) | 0.6 |
| dNTPs | 0.4 |
| Forward primer (50μM) | 0.2 |
| Reverse primer (50μM) | 0.2 |
| Template (10ng/μl) | 0.5 |
| Phusion HF polymerase | 0.2 |
Prepare one or two extra tubes-worth of reagent (you'll use some liquid on the walls of your tips).
Once you've finished, you should run the resulting products on a gel to check if everything went as planned.
Tips
- Thaw the HF-Buffer, DMSO and dNTPs before making the mastermix.
- Avoid taking the Phusion-HF polymerase out of the freezer (only take it out briefly when you need to add it).
- If the reactions have different primers and/or template, add the polymerase right after the dNTPs, split the mastermix and add the rest.
- Don't forget positive and negative controls
- Primers should have similar Tms (less than 5°C).
- Primer Tm calculation is a less exact science than it should be (just test several tools and compare their results). If you're not sure what the correct Tm is, consider using a gradient PCR.
- Avoid primers with strong secondary structures.
- PCR can introduce mutations. Don't forget to sequence your final product (this could be your final plasmid): you really don't want to lose a few weeks because of a "corrupt" plasmid.
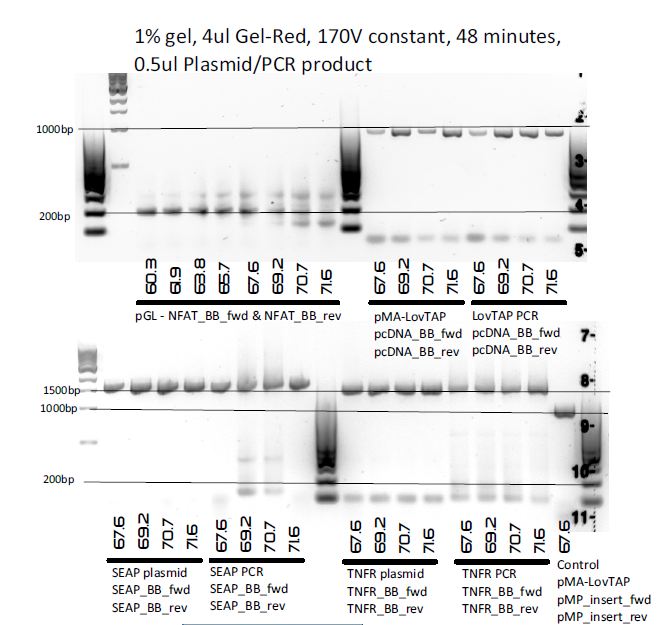
PCR to amplify as much inserts as possible for Biobricking, and to determine once and for all what the correct BioBricking annealing temperatures are.
Templates:
- pGL4.30 (for NFAT)
- pMP-PB-TNFR-Fc
- pMP-PB-SEAP-FLAG
- pMA-LovTAP
Eight 20μl reactions per Biobrick insert were made, so in total 32 main reactions and 1 control reaction (pMA-LovTAP with LovTAP_fwd and LovTAP_rev).
Results
The bands for NFAT are slightly weird, but for the rest, BioBricking PCRs finally seem to work!
pGL backbone plasmid digestion
Protocol: Restriction site digestion
- Look for the best pair of restriction sites, ideally with similar digestion temperatures and times.
- NEBcutter for finding cutting enzymes.
- Double Digest Finder for the parameters.
- Calculate the amounts required of:
- DNA
- Buffer (usually from 10x to 1x)
- BSA, if needed (usually from 100x to 1x)
- Enzymes (depends on the amount of DNA)
- Water
- Get the recommended buffer (and BSA if needed) from the freezer and let defreeze.
- Mix all the ingredients, except DNA, in a tube.
- Note: Enzymes should stay no longer than a couple of minutes out of the freezer. Don't touch the bottom of the tubes! Don't vortex!
- Distribute the mix in as many tubes as DNA samples and add the DNA.
- Keep in the Thermomixer at the recommended temperature.
Sowmya's recommended amounts (50 µl total solution):
- 5 µl of 10x buffer
- 0.5 µl of 100x BSA
- 1 µl of each enzyme
- 5 µl of DNA
- 37.5 (up to 50 µl) of water.
Protocol based on what was done on July the 4th.
The minipreps we had just made were digested with HindIII and MfeI, for ligation with SEAP.
Gel for digested pGL
Protocol: Gel Electrophoresis
Agarose concentration depends on the size of the DNA to be run. We will mostly use 1%.
VOL is the desired volume of gel in ml:
CH Lab
- Add 0.01*VOL g of agarose to a clean glass bottle.
- Pour VOL/50 ml of 50xTAE in a graduated cylinder. Fill up to VOL ml with di water.
- Add the resulting VOL ml of 1xTAE to the glass bottle with agarose.
- Microwave, at 7, the bottle (loose cap!) until it boils.
- Carefully remove bottle (can be super heated!) and check for the total absence of particles. Microwave again if needed.
- Prepare a gel box, with comb, and fill it up with the agarose solution (maybe not the whole solution is needed).
- Add 0.05 µl per ml of gel in the box of Red Gel (it's in the iGEM drawer) and stirr until disolved.
- Wait until cold and solidified.
- Carefully remove comb.
- Place the box in the electrophoresis chamber.
- Fill up the electrophresis chamber with 1x TAE buffer.
- Add blue dye to the DNA samples (6x loading buffer, that is 10 µl in 50 µl of DNA solution).
- Inject 30 µl of ladder marker in the first well (that's 1 µg of DNA).
- Inject 60 µl of each DNA solution in the other wells.
- Set voltage to 70-90 V and run for 30-40 min, or until the dye reaches the last 25% of the gel length (DNA travels from - to +).
- Place the gel under the camera, cover, turn UV on and take photos!
Preparing the ladder:
- get 1kb ladder DNA from the freezer (500 µg/ml).
- for 30 charges, 30 µl per charge, we need 900 µl:
- 60 µl of 1kb ladder DNA
- 150 µl of dye (6x loading buffer)
- 690 µl of water
BM Lab
In this lab the gels are slightly different. The total volumes for the small, the medium and the large gel are respectively 60ml, 80ml and 90ml. As we use 0.5x TAE buffer instead of 1x, we can use higher voltages (170V seems to work fine). The gel should run 20-40 minutes, not more. As the gel is thinner, load less DNA (up to ~10ul).
A gel electrophoresis was performed on the digested version of pGL.
Gel extraction of the pGL backbone
Protocol: Gel Extraction
A gel extraction is used to select a fragment of DNA of a specific length
out of a solution composed of different fragments (ideally the difference
in length between the wanted fragment and the closest-sized fragment should
be more than 200bp). These fragments are often obtained after a
digestion.
The yeild for this procedure is typically very poor so a large amount of starting material, digested DNA in this case is required. We typically used 4 micrograms. The digestion products are loaded on a gel. Lanes on both sides of the one to be extracted should be empty to make cutting easier and avoid contamination with other fragments.
The gel should be run long enough for the bands to be spread out. This is particularly important if the fragment of interest is around the same length as other expected digestion products. UV light is necesary to observe the bands on the gel but exposure time should be minimized to avoid DNA damage. The fragment of interest is then excise and put in an Eppendorf (consider using a 2ml one).
To extract the DNA from the agarose we used Macherey-Nagel's "Nucleospin® Gel and PCR clean-up" kit. Its manual can be found here: Gel and PCR clean-up Manual
Tips
- Cut away as much Agar as possible without slicing into the DNA. Excess agar will require more solvent to dissolve and will result in a poorer yeild upon elution.
- Minimize the DNA's exposure to the UV-light. UV will damage DNA and have negative effects on any subsequent reactions (for example, ligations can be 10'000x less effective when DNA has been exposed to too much UV light [1]
The correct part of the backbone was excised from the gel and then extracted as per the usual protocol.
Nanodrop
Protocol: DNA Concentration Measurement
- Take a 6 µl aliquote of the DNA and put back the main DNA tube in the fridge.
- Go to the room by the E.Coli lab (LBTM, not on Friday morning!) with:
- The 6 µl aliquote
- A 10 µl pipet
- Optionally, the buffer you used for DNA elution (there might be some next to the machine).
- The machine is the NanoDrop Spectrophotometer.
- On the computer, click on "Nucleic Acid".
- Put a 2 µl drop of (nuclease-free) water on the machine's tip as you are asked to and measure.
- Clean tips (both sides) with a quarter of tissue.
- Add 2 µl of the buffer you use and click on "Blank".
- Clean tips (both sides).
- Add 2 µl of your DNA sample and click "Measure".
- Clean tips (both sides) with a tissue.
- Take 2 measurements per sample (for averaging).
- Print the report when you are done
- Click on exit.
The important numbers are:
- 260/280 ratio, must be > 1.8
- 260/230 ratio, must be > 2 (too big, > 2.5? , might mean too much salts)
- Of course the DNA concentration.
The DNA yield for the gel extraction was low, as usual.
Ligation of pGL-SEAP
Protocol: Ligation
Ligation is a method of combining several DNA fragments into a single plasmid. This is often the
step following a PCR (and a PCR cleanup) or a gel extraction. You can also do a "dirty" ligation, where you follow a certain number of digestions directly by a ligation.
- Download the following spreadsheet : File:Team-EPF-Lausanne Ligation.xls
- Fill in the pink areas with the vector and fragment concentration, their size and the ratio.
- Add all the suggested ingredients order in a microcentrifuge tube, in the order they appear.
- Ligate for 2 hours at 14ºC.
- Immediately transform competent bacteria with the ligation product.
Note: This protocol hasn't been optimized for blunt-end ligation (though it might still work).
The freshly gel-extracted pGL backbone and the SEAP digested PCR product were ligated together.
Amp agar plates
Protocol: Agar Plates
- Add to a bottle:
- 20 g/l of LB broth powder.
- 10 g/l of Agar (not agarose).
- Fill up with DI water and autoclave (program 106, takes 2 hours). Remember to leave the cap loose!
- Label the plates (found in the stock room).
- When the autoclaving is done, close the bottle cap and take the bottle out.
- Let rest until it cools down to around 55ºC (can be held for some seconds): if warmer, the antibiotic degrades, if colder, the broth gelifies.
- Add the antibiotic (Ampicillin 100 µg/ml) = 1 ml of 100 mg/ml Amp for 1l of broth. Same for chloramphenicol or Spe.
- Add 25-30 ml of broth to each plate and let open for 2 hours.
- Close plates, wrap in alu foil (Amp is sensitive to light) and store at +4ºC.
New Amp agar plates were made.
 "
"