Team:Wageningen UR/Journal/week17
From 2012.igem.org
week 17: 20 august - 26 august
Office work
Lab work
TuYV
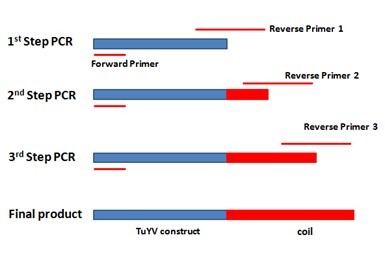
On the basis of successfully getting TuYV VLPs, modifications with two protein coil-structures will be explored on the outside of TUYV VLPs. The two coils, E/K.coil, have electrostatic interaction due to different charge and can serve as an anchor for other proteins. K.coil, which is positive charged, will be attached to the C terminus of the VLPs monomer and E.coil, which is negative charged, will be attached with other proteins, such as using GFP as reporter ligand. For adding the E.coil to the C terminal of monomer, three step extension PCR was designed in order to fuse the coil gene to the 3’ end of our construct gene. Specific reverse primer was designed for each step, totally three. The primer’s 5’ end contains new sequence and 3’ end will bind to the template. After each step PCR reaction, the new sequence will be added to the 3’ end of the template and the PCR products from this step will be utilized as template for the next step. Finally, the 63bp E.coil will be added to the 3’ end of our construct gene. The overall scheme is showed in the figure below.
This week we started adding the coil to the C terminal of TuYV constructs. With designed primers, the first step PCR showed bands on the right sizes. The second step PCR was tried three times this week, but we always did not get any band on the gel check for second step PCR. This was because after the first step, we did gel extraction for our first step PCR products, but the DNA concentration was very low after gel extraction and the template was destroyed during the extraction process.
TuYV constructs in Ampicillin resistant backbone and BBa_J04500 were digested and checked on the gel, but we did not see any band on the gel check for all 4 sample. Later, we loaded the miniprep products on the gel, we got smear, it turned out to be our miniprep products were degraded.
TuYV constructs (PCR procucts: 1, 1H, 4, 4H) were digested, ligated and transformed into JM-109 again, we got very promising plate, there were lot of colonies on the sample plate.
Hepatitis B
20 August
- Colony PCR
- of HepB (without promoter) in BBa_J04450 - of HepB with a his tag + IPTG promoter in Bba_J04500 -> the samples show no band on the gel - so the transformation did not succeed
- Ligation
- of the HepB with the IPTG promoter into BBa_J04450
- Transformation
- Both into JM109 and DH5α; using the BBa_J04450 brick as positive control
22 August
- Colony PCR
- Of the transformations HepB + IPTGpromoter in BBa_J04450 backbone in JM109 and HepB + IPTGpromoter in BBa_J04450 backbone in DH5α -> the samples show no band on the gel - so the transformation did not succeed
23 August
- 2nd try - Colony PCR
Of the transformations GFP-coil in BBa_J04450 backbone in JM109 and GFP-coil in BBa_J04450 backbone in DH5α -> again no band on the gel
Hepatitis B inside modification
23 August
- Step 3 and step 4 of the PCR reaction
-> no bands visible for the 3rd and 4th step of HepBinside-coil
- control of a miniprep of HepB+IPTG in BL21 from 16.Aug (sample from 6.Aug)
-> the miniprep shows a band of the expected size - so the transformation from 6.Aug was successful
24 August
- repeat 3rd step of the PCR reaction with the sample of 15. Aug
-> 3rd step of the PCR reaction seems to work (right size + size difference compared to the 2nd step, but also a smear is visible
GFP modification
20 August
- Ligation
- of the digested GFP-coil PCR product from 15.Aug into BBa_J04450
- Transformation
Both into JM109 and DH5α; using the BBa_J04450 brick as positive control
22 August
- Colony PCR
Of the transformations GFP-coil in BBa_J04450 backbone in JM109 and GFP-coil in BBa_J04450 backbone in DH5α
23 August
- 2nd try - Colony PCR
Of the transformations GFP-coil in BBa_J04450 backbone in JM109 and GFP-coil in BBa_J04450 backbone in DH5α
24 August
- check and amplify GFP-coil PCR product from 14.Aug
-> no conclusion can be made because of very weak bands
 "
"