Team:Evry/Tadpole injection1
From 2012.igem.org
Characterization of plasmids in Xenopus tropicalis
In order to test ours plasmids we injected them into fertilized eggs, then we traced the fluorescent expression throughout time and space depending on promoter (ubiquitous or tissue specific).
A very simple injection tutorial explains with diagrams how to do the injections and how to take care of embryos and tadpoles. The experiment last 5 days, from an unfertilized egg to a swimming tadpole at stage 48-50. The GFP (or any other fluorescent protein) is expressed few hours after the fertilization to the end of the week (see below).
The same protocol was used to inject eggs, and we injected 2.3 nl of plasmid at 100ng×µl-1 - embryos were stored at 21°C during the experiment week.
Embryos and tadpoles grew up with their own vitellus, without the need to be fed during the week of experiment. Pictures were taken with the Zeiss stereomicroscope: SteREO Lumar V12 with the camera AxioCamMRm.
The iGEM-Evry tem say a great thanks to Dr. Nicolas Pollet, Dr. Aurore Thelie and Lena Vouillot (PhD student) who taught us how to inject embryos, take care of tadpoles and how to use their microscope. They are from the Institute of Systems & Synthetic Biology of Evry in the Metamophosys group.
Plasmids injected:
- pCS2+ Elastase/sfGFP: This plasmid contains the tissue specific promoter elastase and the fluorescent protein sfGFP, elastase is a promoter specific to pancreas. This Biobrick created by our team is BBa_K812233 (ready to use in Xenopus). This plasmid was built from the eukaryotic plasmid (with elastase promoter) BBa_K812200 and the BioBrick sfGFP (with the Kozak sequence) BBa_K812031. Number of Plasmids injected: ~ 5.27E+7
- pCS2+ GFP-aid: This plasmid contains the constitutive promoter CMV and the aid sequence of the aid system fused to GFP (Green Fluorescent Protein)(Nishimura et al., 2009). This Biobrick created by our team is BBa_K812110 (ready to use in Xenopus). This plasmid was built from our pCS2+ eukaryotic plasmid BBa_K812000 with the BioBrick GFP-aid BBa_K812010. Number of Plasmids injected: ~ 3.78E+7
- pCS2+ mCitrine: This plasmid contains the constitutive promoter CMV and the fluorescent protein citrin (yellow). The plasmid BBa_K812130 (ready to use in Xenopus) is from our pCS2+ eukaryotic plasmid BBa_K812000 with the BioBrick mCitrine (with the Kozak sequence) BBa_K812030. Number of Plasmids injected: ~ 4.38E+7
- pCS2+ mCFP: This plasmid contains the constitutive promoter CMV and the fluorescent protein mCFP (Cyan Fluorescent Protein). The plasmid BBa_K812132 (ready to use in Xenopus) is from our pCS2+ eukaryotic plasmid BBa_K812000 with the BioBrick mCFP (with the Kozak sequence) BBa_K812032. Number of Plasmids injected: ~ 4.37E+7
- pCS2+ sfGFP: This plasmid contains the constitutive promoter CMV and the fluorescent protein sfGFP (super folded Green Fluorescent Protein). The plasmid BBa_K812133 (ready to use in Xenopus) is from our pCS2+ eukaryotic plasmid BBa_K812000 with the BioBrick sfGFP (with the Kozak sequence) BBa_K812031. Number of Plasmids injected: ~ 4.37E+7
Conclusion
Our constructions were expressed in embryos and then in tadpoles. Presented BioBrick parts are considered as characterized. Nevertheless we expected an uniform expression of reporter with the CMV promoter. Colors fluorescent proteins were expressed in different tissue, one tadpole expresses the colors fluorescent protein in one to four different tissues, and tissue are different between tadpoles.
Hypothesis: The plasmid does not diffuse in the egg and stay in the same area, it means that depending on the injection area the plasmid would be in a part of the tadpole. This question was raised in our model. Another reason could be that the metabolism of Each tissue specific cell is different and change during the tadpole's development.
More experiments needs to be conducted to confirm that we showed the tissue specificity of the elastase promoter (pancreas). Four different reporters were characterized: mCitrine, CFP, sfGFP and the fused protein GFP-aid.
Moreover the expression of reporters decreases throughout time, because plasmids are damaged throughout times but also because plasmids are shared between more cells and the quantity of plasmids decreases for each cell.
Plasmid injection results
pCS2+ Elastase/sfGFP
Tadpoles are at stage 47. Their size is near 5 mm.
We can see a sfGFP expression in a region close to the intestine and the biliar vesicle, and it is not auto-fluorescence. We suspect it to be the pancreas but as it is small in the tadpole it is not easy to testify. This injection will be done again in order to confirm our results.
pCS2+ GFP-aid
24h after injection
Embryos are around stage 20. The neural fold is visible and the size of neurulas is near 1 mm
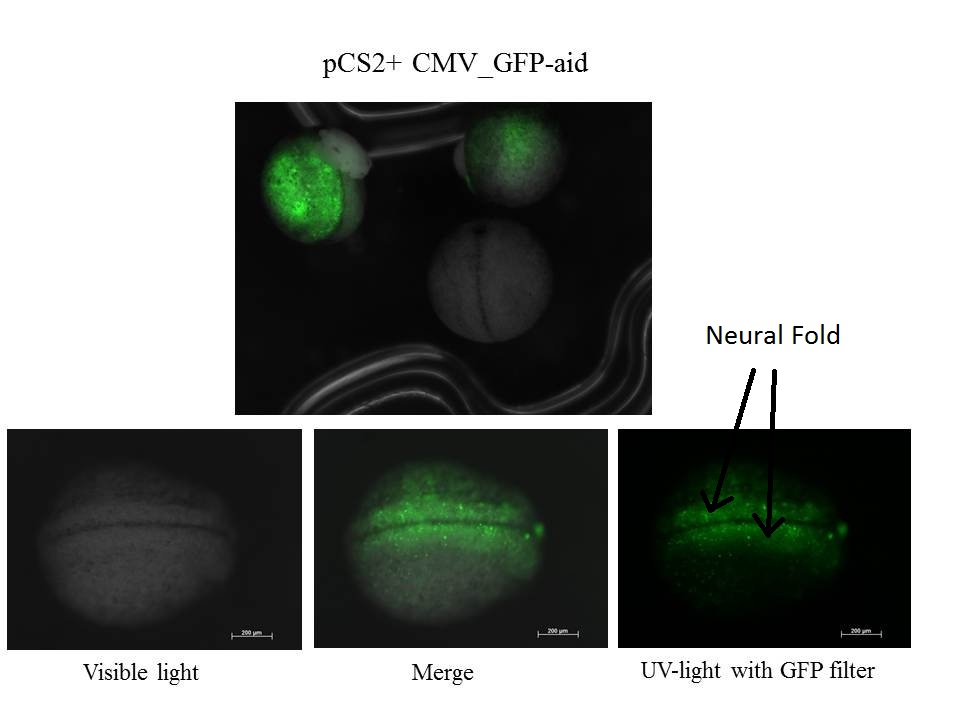

z-stack of the embryo
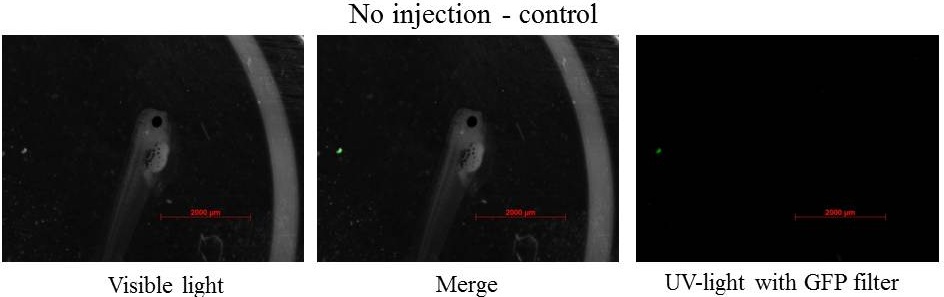
Some eggs expressed GFP-aid and some others did not meaning that the injection did not work for them or the GFP-aid was not express
48h after injection
Embryos are at stage 34-38 and move by intermittence, the size of tadpoles is near 2.5 mm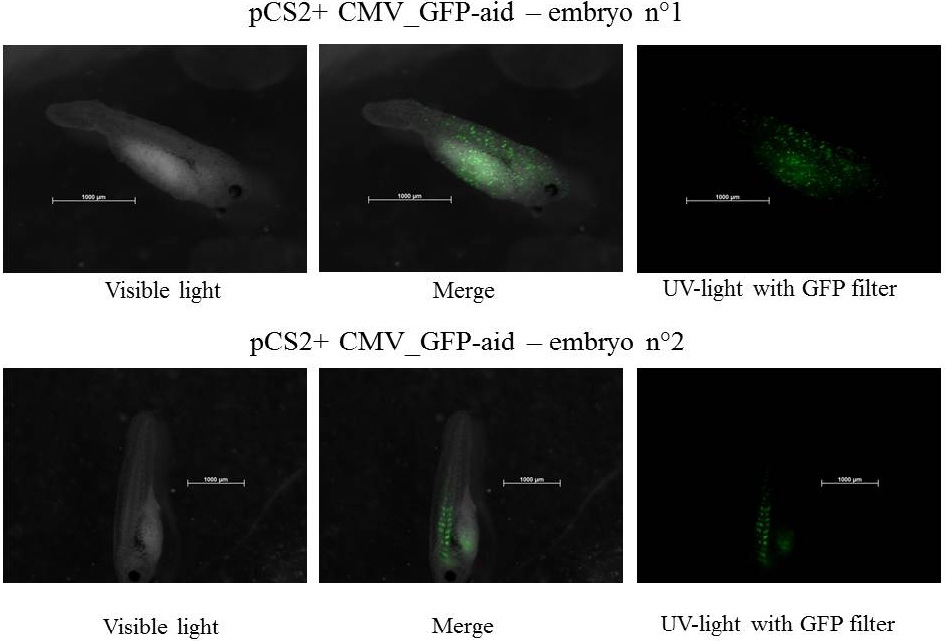
Despite a constitutive promoter in the plasmid (CMV), the expression of GFP-aid is localized in different tissues for each tadpole. We can hipothesize that the plasmids do not diffuse in the eggs because of the vitellus viscosity. This question was raised in one of the modelling part.
3 days after injection
Embryos are at stage 41-42. They swim and their size is near 4 mm.From this tadpole stage an anaesthetic is required to take pictures of tadpoles, otherwise the light teases tadpoles, and it is impossible to take a picture.

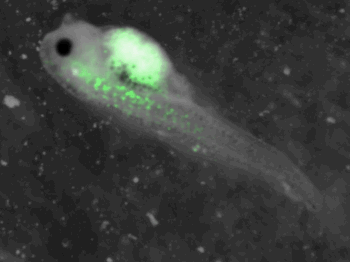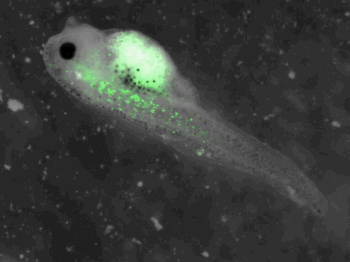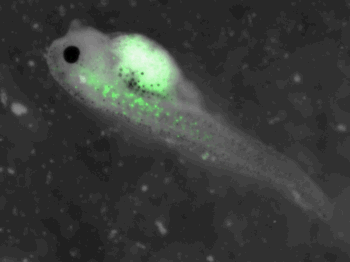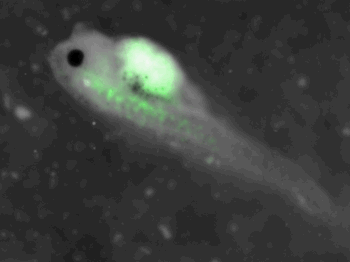
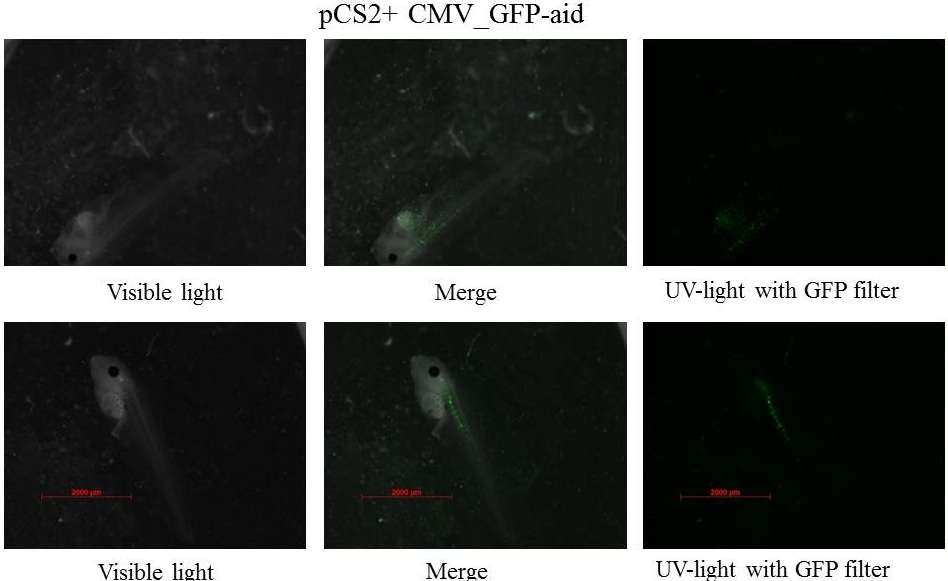
z-stack pictures: pCS2+ CMV_GFP-aid, GFP expression is not localized in same tissue between tadpoles. For instance in the tadpole on the left picture, bones in the tail produced GFP; on the right picture the GFP expression is localized in the skin. In this animation the only part of the tadpole moving is the heart beatting (between the head and the stomac).
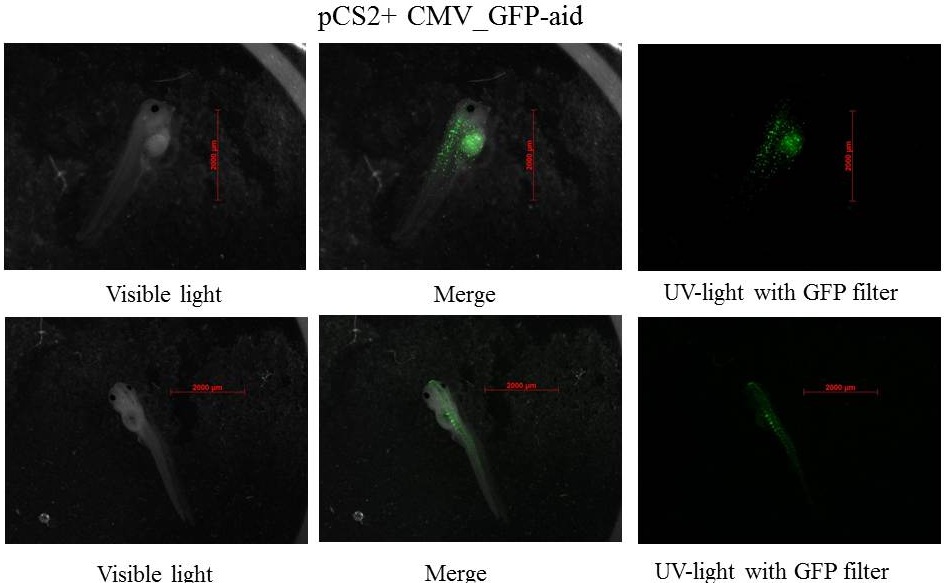
The expression of GFP-aid is localized in different tissues for each tadpole, like the day before. GFP is present in same tissue, it means that plasmids stay in same cells.
4 days after injection
Embryos are at stage 45-46. They swim and their size is near 5 mm.
The GFP is still present in specific tissue but the intensity of the signal is decreasing. Plasmids may be damaged by cells, and/or the quantity of plasmids may have decrease in each cells which involded the diminution of GFP in each cells, after that it is more difficult to see GFP. Picture with the LSM 510 META Laser Scanning Microscope from Zeiss. A great thank to Dr Daniel Stockholm and Genethon for using this microscope.
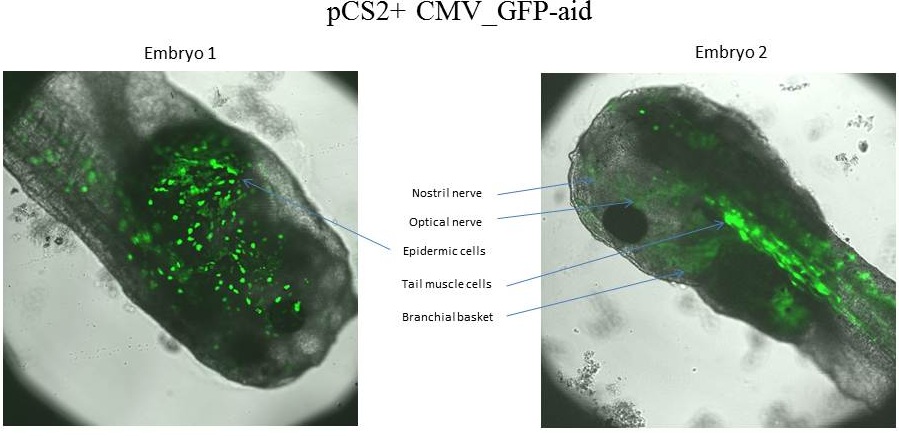
The tadpole 1 express GFP-aid in epidermic cells, whereas the tadpole 2 express GFP-aid in optical nerve,nostril nerve, tail muscle cells and branchial basket.
pCS2+ mCitrine
Tadpoles have 4 days (stage 45-46), because our lab does not have YFP filter for the mCitrine fluorescent protein the LSM 510 META Laser Scanning Microscope from Zeiss was used.
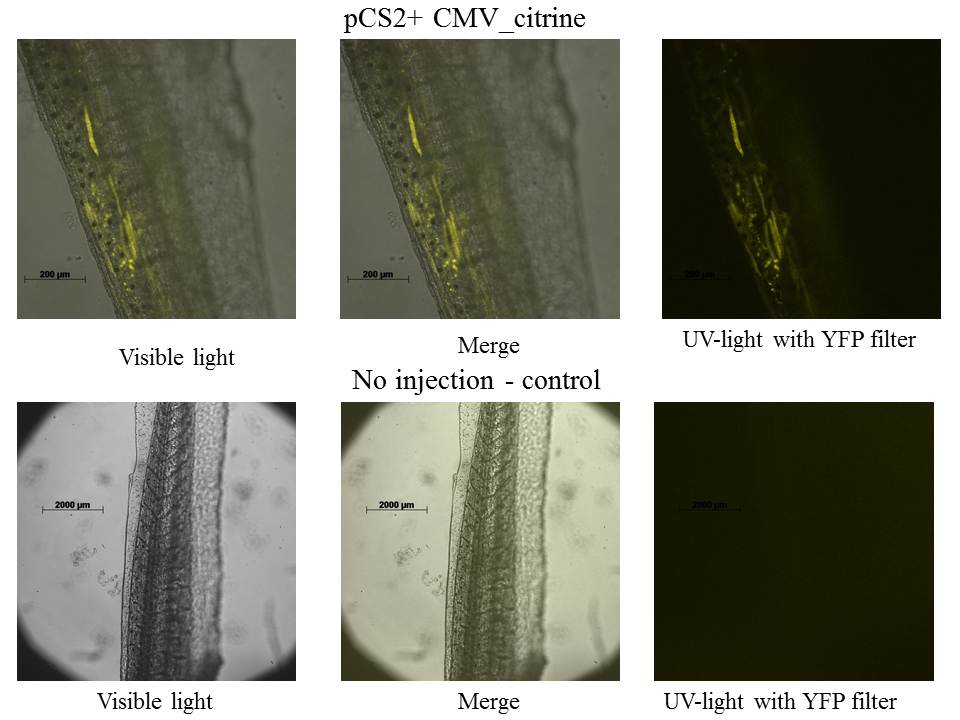
The expression of mCitrine is localized in tail's muscles.
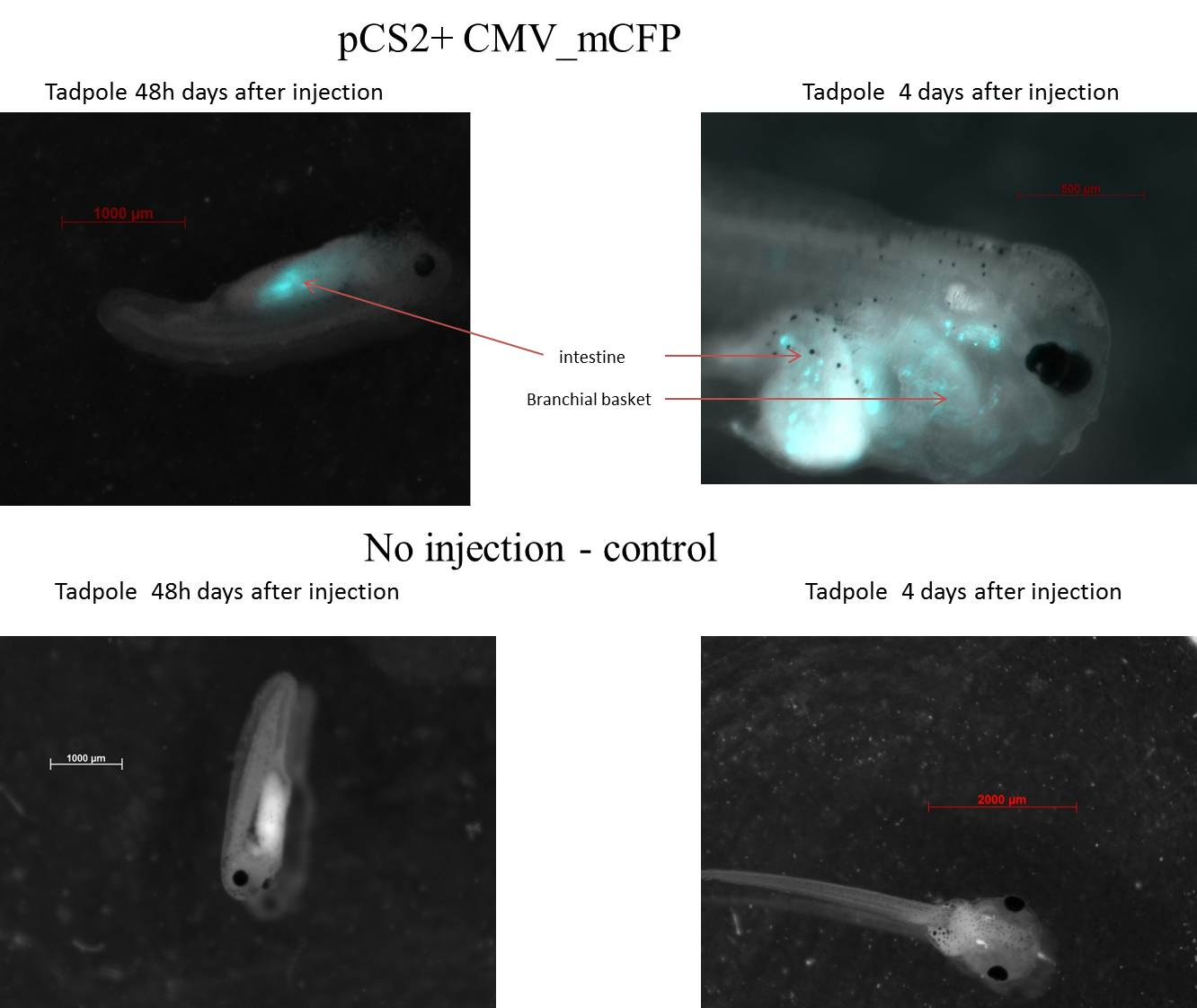
pCS2+ mCFP
Tadpole 1 is 2 days (stage 36-38), the expression of CFP is localized in intestine. Tadpole 2 is 4 days (stage 45-46), the expression of CFP is localized in Branchial basket and intestine.
pCS2+ sfGFP

The expression of sfGFP is present only in one out of the two embryo/tadpole. sfGFP is present only in few tissue as others reporters. sfGFP is expressed in tail's muscles and branchial basket for this tadpole.
messenger RNA injection
Xenopus embryos were injected with mRNA, obtained from in-vitro transcription of our frog plasmid using a commercial kit.

We can see a strong expression of GFP in all tissues, proving that our in-vitro transcription primer works. This debugging tool can be used when no expression is visible in the tadpole: It allows to be certain that the problem does not come from transcription. If RNA is injected and no activity is observed, then the bug is probably due to problems downstream of transcription (no translation, rapid degradation or incorrect protein folding for example).
We can note that the green fluorescence is much better spread throughout the embryo than it is with DNA. This is because the messenger RNA diffuses much better throughout the Xenopus embryo.
References:
- Inducible control of tissue-specific transgene expression in Xenopus tropicalis transgenic lines., Chae J., Zimmerman L.B., Grainger R.M., Mechanisms of development 117:1-2, 2002
- Xenopus: a prince among models for pronephric kidney development., Jones E., JASN 16:2, 2005
- An auxin-based degron system for the rapid depletion of proteins in nonplant cells, Nishimura K., Fukagawa T., Takisawa H., Kakimoto T., Kanemaki M., Nature Methods 6:12, 2009
 "
"