Team:TU Darmstadt/Protocols/TEM-EDX
From 2012.igem.org
TEM-EDX-Measurement
[http://en.wikipedia.org/wiki/Transmission_electron_microscopyTransmission Electron Microscopy] is a mode of electronic microscopy where a electron beam is focoused an transmitted on a ultra thin probe (<100nm). The electron interaction of the whole thickness of the sample can be measured.
[http://en.wikipedia.org/wiki/Energy-dispersive_X-ray_spectroscopy Energy Dispersive X-ray spectroscopy] is an analytical technique to investigate the elemental composition and chemical characterization of a sample. It is based on the characteristically dispersion specra of an electron beam by each element. This method allows to identiy which elements are present in a measured sample.
If the TEM method is coupled with EDX its now possible to identify the specific element composition in the whole layer thicknes of a sample.
Experimental Set Up
After sample preparation we put it into an electron microscope and radiated with an electron beam. Due to the radiation electrons from the inner shell of the atoms in the probe are driven out and create an electron hole. While electrons from the an outer shell fall down into this hole they radiate an energy spectra. This spectra is characteristic for every element and gives us the total composition of the radiated probe. We use EDX measurements to proof the functionality of our chosen tricarboxylat tripartiate transporter. If the transporter is functional the composition of C and O atoms in the cytosol of our microorganism must be different from probes without the transporter.
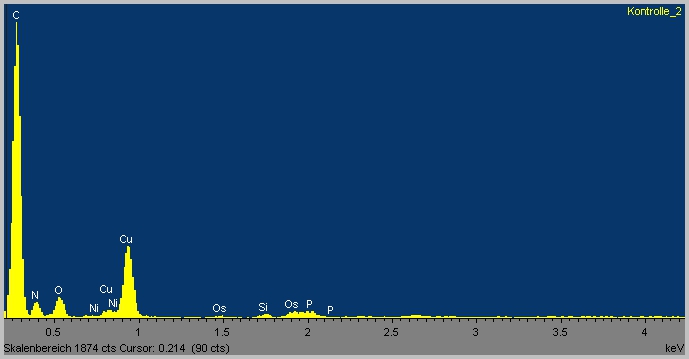
Detected Spectra
The spectra show a clear difference between background and sample measurement. In the background spectra no nitrogen (N) and phosphor (P) occurs. These elements are perfect markers for biological material. Although there is nor clear evidence for the uptake of TPA into the cells the TEM-EDX experimental set up is a good method to achieve this goal. Unfortunately time is short in the iGEM competition, otherwise we would add a heavy atom like bromide or chloride to TPA. As seen in the spectra non of this elements occur in our sample, which would make it possible to clearly identify the source of a Br/Cl peak as TPA.
 "
"