Team:TU-Eindhoven/Future applications/Developments
From 2012.igem.org

Device
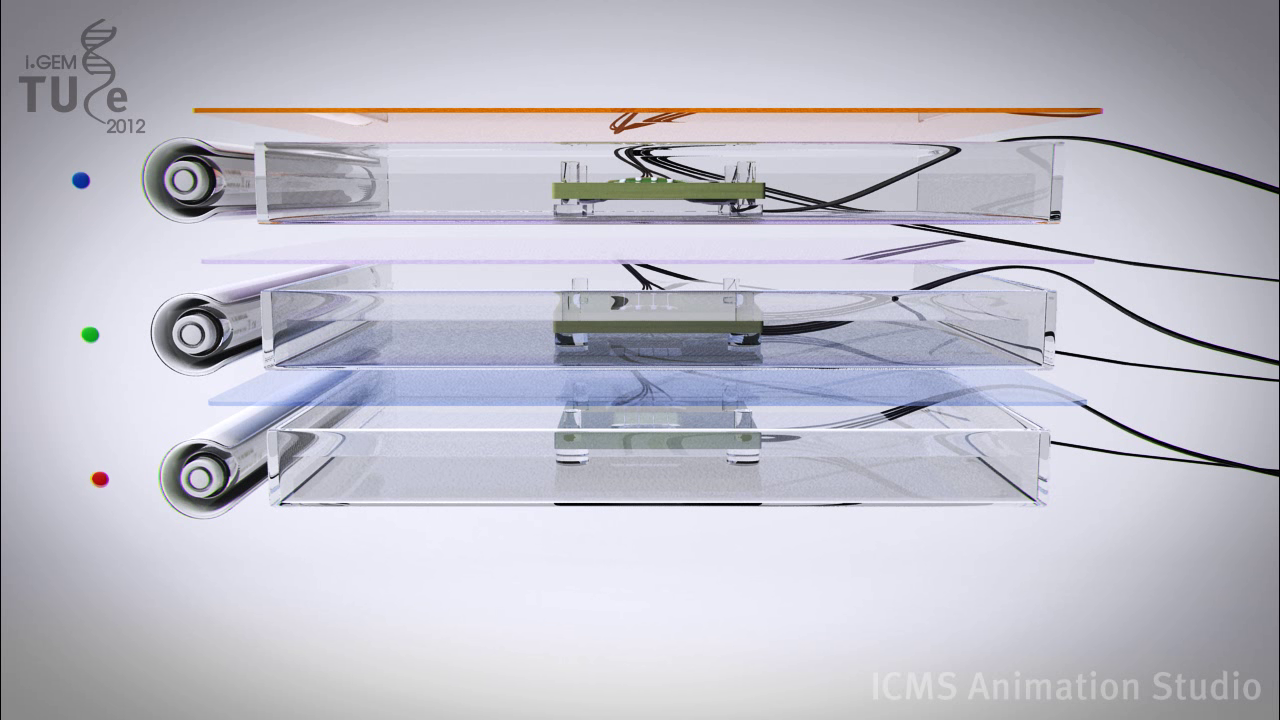
As said in the device part of the wiki, the resolution of the screen is only limited to your choice of electrodes. Thus creating a high definition screen should not be hard at all. More difficult is adding colors to the screen. Every screen nowadays has a color display, from the smartphones to the huge LED TV screens.
The design for our color screen needs of course the three colored yeasts. The device would then consist of three medium baths (one for each yeast). These baths would each have an appropriate light source at the most efficient wavelength for the GECO protein. Also each bath will have a filter on the top that filters away the light source, thus leaving only the fluorescent light from the GECOs. The colors have to be stacked (from bottom to top) as red – green – blue. It has to be this specific order, because in any other order the GECO could absorb the light from an underlying layer, thus preventing one color to come through to the top. With this device you can create a high definition color screen or SOMY-LCD.
Model
To improve the model for the aim of the project, the parameters in the model will be adapted, using experimental data, to predict the calcium dynamics in yeast cells. A complete sensitivity analysis needs to be applied, in order to determine the best settings for the experiments, i.e. the concentration of the extracellular Ca2+ concentration.
Furthermore, the influence of calcium diffusion through the cell could be taken into account in an extended model. Instead of MATLAB, COMSOL simulation software can be used, because it is less complex to model dependency on both space and time in COMSOL. Subsequently, the results of the model will be compared to the results of the experiments.
Lab
The more research that is conducted with yeast, the more informed and controlled future research can be. Yeast is incredibly flexible, it is safe and yeast also shares many genes with human cells. So if you want to find out what a particular drug does to a certain human gene, you can often test it on yeast cells first.
A good way to characterize the whole yeast cell is still missing. This would be useful to validate the computational models and to ‘debug’ the yeast. However, this is a lot of work, because every part of the characterization is already a project in itself. For instance:
- The localization of the channels and the control of that. This can be done with fluorescence microscopy, because there are fusion proteins available from CCH1 and Mid1 with EGFP[1]. It is also possible to influence the localization within the cell, this is currently a major area of research in cellular biology.
- The measurement of the dynamic response of the yeast on the calcium influx. This can be done with fluorescence-lifetime imaging microscopy, to measure calcium concentrations in living cells at multiple scales using lifetime contrast. However, only if you are in possession of such an expensive device and if you are able to work with it.
- Optimization of protocols for the cultivation of the yeast, with an optimal calcium regulation. These protocols can be used for measuring the expression of calcium channels and measure the set-point concentration of calcium in the periplasm, ER, Golgi, and vacuole medium.
- Over expression of calcium pumps in yeast. There is still uncertainty about the export mechanism of calcium in yeast cells. To make sure that the calcium does not accumulate in the cell, to get a good response time, over expression of calcium pumps is desirable.
It was also very time consuming to transform three insert, one by one, in yeast. A better approach would be to put all inserts in one shuttle vector of E.coli, with different promoters to regulate their expression. This would decrease the waiting time because the growth rate of yeast cells is not more than 1/3 of the growth rate of E.coli.
Beside those future developments there is always more room for improvements and developments.
References
- [1] K. Iida, et al., Essential, completely conserved glycine residue in the domain III S2S3 linker of voltage-gated calcium channel α1 subunits in yeast and mammals, Journal of Biological Chemistry 282: 25659-25667, (2007)
 "
"