Team:Evry/Protocols
From 2012.igem.org
Chr.karine (Talk | contribs) |
Chr.karine (Talk | contribs) |
||
| Line 161: | Line 161: | ||
Fold the flaps and skin incision in the muscular wall avoiding severing the abdominal vein to avoid hemorrhage. Both gonads are located in the ventral face of kidneys. <br> | Fold the flaps and skin incision in the muscular wall avoiding severing the abdominal vein to avoid hemorrhage. Both gonads are located in the ventral face of kidneys. <br> | ||
Both gonads are located in the ventral surface of the kidneys, they cover more or less and in corpora lutea (fat mass).By extracting them from the abdominal cavity, we can extract the testicles attached to them. The male's testicles are two oval organs, smooth, yellow-pale as can be seen in the photograph below.<br> | Both gonads are located in the ventral surface of the kidneys, they cover more or less and in corpora lutea (fat mass).By extracting them from the abdominal cavity, we can extract the testicles attached to them. The male's testicles are two oval organs, smooth, yellow-pale as can be seen in the photograph below.<br> | ||
| + | Roll the testicles on kitchen paper to remove blood and blood vessels. Dip each testicle in a tube of 1,5mL with 250µl of L15/FCS. Keep tubes on ice. Crush the testicles with a pestle until obtaining a homogeneous solution. Add 250µl of L15/FCS, mix with a P1000 always on ice.<br> | ||
| + | |||
| + | <h2>Lay eggs of females<h2> | ||
| + | Spawn females (who have already started laying alone) in Petri dishes previously coated with L15/FCS (gently massage into their flanks).<br> | ||
| + | |||
Revision as of 14:38, 8 August 2012
Contents |
PCR with Phusion High-Fidelity DNA Polymerase
Tube preparation
Put items in this order:
| Component | 50µl reaction | Comments |
| H2O | 32 | |
| 5x Phusion HF Buffer | 10 | |
| 10mM dNTPs | 1 | |
| Primer FW | 2 | Primers have to be at 10µM |
| Primer RV | 2 | Primers have to be at 10µM |
| Template DNA | 1 | |
| DMSO (optional) | 1,5 | recommended for GC-rich amplicons < 20kb |
| Phusion DNA polymerase | 0,5 |
Cycling instructions
| Cycle step | Temperature | Time | Cycles |
| Initial denaturation | 98°C | 4min | 1 |
| Denaturation | 98°C | 20s | 30 |
| Annealing | Lower Tm of primers | 30s | |
| Extension | 72°C | 30S/kb | |
| Final extension | 72°C | 10min | 1 |
| 4°C | hold |
Préparation of LB medium and LB Agar:
=> LB Agar :
-18,5g LB Agar
-300ml H2O
=> LB medium : -6g LB broth -300ml de H2O
Autoclaved at 250°C
Gel extraction
1. Excise the DNA fragment from the agarose gel with a clean, sharp scalpel under UV light.
2. Weight the gel slice in a colorless tube. Add 3 volumes of Buffer QG to 1 volume of gel.
3. Incubate at 50°C for 10 min, until the gel slice has completely dissolved.
4. The color of the mixture have to be yellow, otherwise add 10 µl of 3M sodium acetate.
5. Ass 1 gel volume of isopropanol to the sample and mix.
6. Place a spin column in a provided 2 ml collection tube.
7. To bind DNA, apply the sample to the column and centrifugat for 1 min. Discard the flow-through and place the column back into the same tube.
8. To wash, add 0,75 ml of buffer PE to the column and centrifugate for 1 min.Discard the flow-through and place the column back into the same tube.
9. Centrifugate the column in a 2 ml Collection tube for 1 min 17,900xg (13,000 rpm).
10. Place the column into a clean 1,5 ml microcentrifuge tube.
11. to elute DNA, add 15 µl of water to the column and centrifugate for 1 min.
In vitro fecondation of Xenopus tropicalis
2 or 3 days before the IVF
Pre-injection of frogs (males et females)
100µl of hCG (0,1UI/µl, do a dilution by 10 of the commercial solution)
D day
Test the injector before beginning
Inject the 100µl hCG (1UI/µl) in the morning
Isolate the animals : an animal by box
Prepare :
Timer
Methylcellulose (put at room temperature)
Boxes special for injections
Materiel of injection
Capillaires ad hoc with oil
Solutions to inject (add 1µl of blue or red of Nile)
Solution L15/FCS :
- boxes (1/femelle) of Pétri (d=10 cm) lined of L15/FCS (500µl)
- 2 tubes 1,5mL with 250µl of L15/FCS (one for each testicle)
Pestles in plastic cleaned to grind the testicles
MMR 0,1X
MMR 0,05X
MS-222 0,2%
Euthanasia of the male
Put the male in a vat with MS-222 0,2% and look forward to his non response of pinches. Put it out and process of the dissection to extract the two testicles.
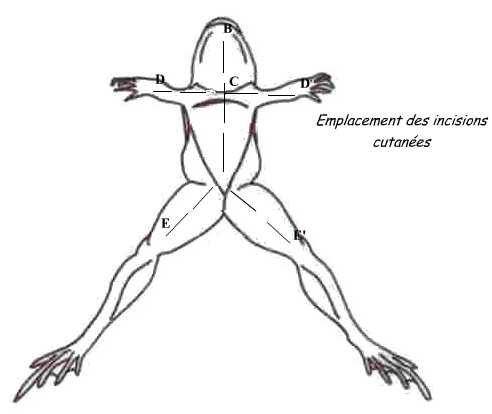
For that, dispose the animal: the dorsal face against the dissection board. Pinch the skin in the middle of the belly and do a small incision with scissors; from this point, perform skin incisions shown in the diagram.
Fold the flaps and skin incision in the muscular wall avoiding severing the abdominal vein to avoid hemorrhage. Both gonads are located in the ventral face of kidneys.
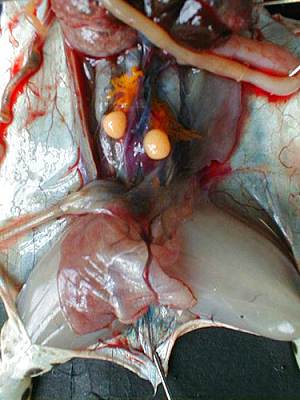
Both gonads are located in the ventral surface of the kidneys, they cover more or less and in corpora lutea (fat mass).By extracting them from the abdominal cavity, we can extract the testicles attached to them. The male's testicles are two oval organs, smooth, yellow-pale as can be seen in the photograph below.
Roll the testicles on kitchen paper to remove blood and blood vessels. Dip each testicle in a tube of 1,5mL with 250µl of L15/FCS. Keep tubes on ice. Crush the testicles with a pestle until obtaining a homogeneous solution. Add 250µl of L15/FCS, mix with a P1000 always on ice.
 "
"