|
|
| Line 22: |
Line 22: |
| | | | |
| | </div><div id=note> | | </div><div id=note> |
| - |
| |
| | </html> | | </html> |
| | + | '''August 2''' [[User:Ruichen|Ruichen]] |
| | | | |
| - | | + | Field trip to Chevron Burnaby site! |
| - | | + | |
| - | | + | |
| - | ==June 25==
| + | |
| - | | + | |
| - | Designed primers for SDM of dszB gene
| + | |
| - | | + | |
| - | PCRed yddG and Try A with Marianne
| + | |
| - | | + | |
| - | Had a Skype meeting with Calgary iGEM team (details can be found in '''Team Collaboration''' section
| + | |
| - | | + | |
| - | -[[User:Ruichen|Ruichen]]
| + | |
| - | | + | |
| - | ==June 26==
| + | |
| - | | + | |
| - | Designed primers for SDM of dszC gene
| + | |
| - | | + | |
| - | Joined Jacob and Joe for transformation of Heat(chemical) competent cells:
| + | |
| - | | + | |
| - | {| class="wikitable"
| + | |
| - | |-
| + | |
| - | | Plasmid transformed||Volume of Ligation Mixture used (µL)
| + | |
| - | |-
| + | |
| - | |dszB, dszC (3 to 3 ratio, Old and New)||3
| + | |
| - | |-
| + | |
| - | |MetA, TrpA, TrpB, TryA, ArgC, positive and negative control||7
| + | |
| - | |}
| + | |
| - | | + | |
| - | -[[User:Ruichen|Ruichen]]
| + | |
| - | | + | |
| - | | + | |
| - | ==June 27==
| + | |
| - | | + | |
| - | Run the gel of Try A and yddg genes at 95 Volts for 1 hr and 30 min, with 1Kb ladder.
| + | |
| - | | + | |
| - | -[[User:Ruichen|Ruichen]]
| + | |
| - | | + | |
| - | ==June 30==
| + | |
| - | In the last few days, we have confirmed that our restriction enzymes work, that our electroshock cuvettes do not due to corrosion from bleach, and still have had no confirmed biobricks. We picked another candidate colony from the ArgE plate because the PCR of the plasmid between VF2 and VR (standard biobrick sequencing primers) was only about 200 bp, while we were expecting about 1.2 kb. We got the same result from our potential MetA colony. We received new ligase, and if that doesn't work, we are going to try the gibson assembly method. Using a previous restriction digest, we attempted another ligation of our new potential biobricks.
| + | |
| - | | + | |
| - | The first set of chemically competent cells did not grow anything. The procedure was repeated and completed today. The cells were transformed with a known plasmid and appropriately plated.
| + | |
| - | | + | |
| - | Rafael gave us 4 new electroshock cuvettes to temporarily replace the ones that we had destroyed. Joe used 3 of them to test his knockout, and as of today, there is one candidate colony growing on a 1/4 antibiotic concentration plate. A battery of tests have yet to be run to confirm that it is a knockout.
| + | |
| - | | + | |
| - | Yesterday, we went to Dr. Ramey, who had taught students who made a successful knockout using the lambda red system. They used a different recombineering plasmid than we did, and we received this plasmid. It was, however, growing in a strain that we were unfamiliar with, and we decided to isolate the plasmid and transform our Epi300 cells with it.
| + | |
| - | It is currently growing in a culture at 30°.
| + | |
| - | | + | |
| - | We received only the TrpB gene from the registry, and successfully grew it on a plate. We are now growing a culture to isolate the plasmid and if Joe's knockout is indeed a knockout, we can characterize the existing biobrick part by complementation.
| + | |
| - | | + | |
| - | We ordered the Gibson assembly kit, and it ran upwards of 700 dollars for 50 reactions.
| + | |
| - | | + | |
| - | Joe successfully PCR'd out the DszD gene from Rhodococcus genomic DNA that he isolated.
| + | |
| - | | + | |
| - | We made Tet, Kan, and Amp plates with 1/2 and 1/4 antibiotic concentration to try growing our knockouts. There was no growth from a resistance-less K12 cell on any of the plates tested.
| + | |
| - | | + | |
| - | Our PCR for the yddg and tyrA genes was unsuccessful.
| + | |
| - | | + | |
| - | -Jacob
| + | |
| - | | + | |
| - | ==July 1==
| + | |
| - | There are many (hundreds) of colonies on Joe's recombineering plates, and the concentration of colonies goes up as the concentration of antibiotic goes down. It appears that the addition of arabinose to the culture did nothing to increase recombineering efficiency. There are several large, well defined colonies on the full antibiotic plate. It appears that 2 days are required for sufficient growth at 30°.
| + | |
| - | | + | |
| - | With this success in mind, I PCR purified a couple of other knockouts (ArgE Tet and TrpB Kan) and transformed them, reusing the electroshock cuvettes we borrowed from Rafael. A pack of 50 was ordered so we could do more than 4 transformations per day. They are currently growing in the 30°C room on the second floor.
| + | |
| - | | + | |
| - | We only had one chlor plate left, and 4 potential biobricks which were to be put on the chloramphenicol resistant pSB1C3 plasmid. Since there was only one plate (and one electroshock cuvette), only the MetA biobrick ligation product was transformed.
| + | |
| - | | + | |
| - | The pKD46 recombineering vector that we received from Dr. Ramey grew well overnight at 30°C, and was miniprepped, and then transformed into the Epi300 strain. The recovery period was done at 30°C as well. If the transformation is successful, Ting will make electrocompetent cells out of the Epi300+pKD46.
| + | |
| - | | + | |
| - | -Jacob
| + | |
| - | | + | |
| - | ==July 7==
| + | |
| - | | + | |
| - | In the past couple of days, we have found candidate knockouts for all relevant genes but trpA, and attempted to confirm that they were all actual knockouts by PCR. Unfortunately, none of the PCR's worked. We suspect that it was because of a bad enzyme, so some of the reactions were repeated. Currently, the results are inconclusive as to whether or not the knockouts are actually knockouts. We also ordered the relevant knockouts from the Keio collection.
| + | |
| - | | + | |
| - | Colonies with potential biobricks of trpA, tyrA, argE, metA, and dszD were successfully grown. This time, chemically competent cells were used, and they were plated on 1/2 concentration chlor plates. We also transformed and grew out the previously characterized minC biobrick.
| + | |
| - | | + | |
| - | To physiologically characterize the knockouts, we made M9 plates, and plan to set up an amino acid gradient. Sadly, we ran out of M9 salts before we could make tet plates, so some knockouts can't be tested in this manner yet. We also made spec plates to grow some things from the registry, but a lawn grew on the negative control. Looking into the literature, it looks like the concentrated antibiotic we were using was not the correct strength.
| + | |
| - | | + | |
| - | PCR of the yddg gene failed yet again. It is possible that the primers are not correct.
| + | |
| - | | + | |
| - | -Jacob
| + | |
| - | | + | |
| - | | + | |
| - | ==July 9==
| + | |
| - | | + | |
| - | Does anybody else update the wiki these days?
| + | |
| - | | + | |
| - | We have been in contact with Calgary regarding kill switches. More details to follow later.
| + | |
| - | | + | |
| - | One of our potential knockouts and a positive control were grown on the M9 plates. There was growth on both plates, although the growth on the positive control was much faster and greater than the potential knockout. Since the potential knockout was able to grow on the amino acid-less M9 media, it appears to be not auxotrophic. Characterization with PCR continues to be difficult, and it was suggested that we simply sequence the strains.
| + | |
| - | | + | |
| - | The plates with the potential knockouts were placed in the 37° incubator overnight to cure the plasmid.
| + | |
| - | | + | |
| - | The potential biobricks were successfully miniprepped, and a PCR was performed to isolate what was actually between the VF2 and the VR primers in the plasmid. The product will then be run on a gel, and if it appears the correct length, will be sent for sequencing.
| + | |
| - | | + | |
| - | Our Pseudomonas competent cells appear to work (conferring antibiotic resistance), but they are not expressing rfp.
| + | |
| - | | + | |
| - | We made spec plates, but a lawn grew with our transformed cells. Interestingly, on top of this lawn grew several well defined colonies. We attempted to grow these colonies (and the proper negative controls) in varying amounts of spectinomycin. It is possible the plates had insufficient spec, or that the strain we are working with is naturally resistant (there was a lawn, but no large colonies, on the negative control).
| + | |
| - | | + | |
| - | Several more biobricks from the registry were transformed.
| + | |
| - | | + | |
| - | Joe made M9 plates with an amino acid, to further test his knockout.
| + | |
| - | | + | |
| - | -Jacob
| + | |
| - | | + | |
| - | ==July 10==
| + | |
| - | | + | |
| - | The PCR to isolate and amplify the insert from the potential biobrick showed many bands, characteristic of non-specific primer binding. As another test to see if the insert was correct, we cut with EcoRI and PstI to look for the appropriate insert size, and indeed found that there was an insert at about the right size. With this in mind, simply sequencing from the plasmid is the next step. However, our sequencing company only accepts orders until 1:30 PM, and it was 4:30 before everything that needed to be done was done.
| + | |
| - | | + | |
| - | The transformations from yesterday's transformations appeared to work. Also, interestingly, the colony from on top of the spec plate lawn, but not the spec plate lawn itself, was able to grow in 40 and 80 ug/mL spec.
| + | |
| - | | + | |
| - | Cameron reports knockouts from Yale.
| + | |
| - | | + | |
| - | -Jacob
| + | |
| - | | + | |
| - | ==July 15==
| + | |
| - | | + | |
| - | Sent the potential biobricks for sequencing last Friday, ordered primers for mutagenesis on DszC, ordered primers for attachment of fluorescent genes to amino acid genes, received all but yddg knockouts from Yale, found that the Arg knockouts work as described. Several other primers were also ordered, but I am not privy to their nature.
| + | |
| - | | + | |
| - | An attempt at doing an experiment to calibrate the fluorescent proteins for the plate reader (looking for quenching, linear relationship between OD and fluorescence, etc) was done on Sunday, but as it turns out, we had the wrong promoter for a fluorescent protein, and the wrong antibiotic resistance for one of the other proteins. Given this, we gave up, and decided to do it later with the proper strains.
| + | |
| - | | + | |
| - | Recently, there was a meeting with the kill switch team from Calgary. They suggested doing more sequencing, and that is indeed a good idea if we want to pursue this project. I think that the intein primers were ordered, but again, I am not privy to exactly what was ordered.
| + | |
| - | | + | |
| - | Competent cells were made from each of the knockouts, and they worked well. The yddg strain was ordered through collaboration with Calgary as well as the keio collection proper in Japan.
| + | |
| - | | + | |
| - | Today, I watched two pairs of eyes. One was black, and the other grey. And while the owners thereof, for the space of five seconds, walked past each other, the grey shot at the black, and the black fiddled the grey. Bonus points for identifying the poem without using a search engine.
| + | |
| - | | + | |
| - | Our Gibson assembly kit came! Doing the math, it turns out that it is about 30 times more expensive than gold, so we will use it wisely. Personally, I would have liked to just order the enzymes and buffer reagents, but c'est la vie. Our biobrick assembly kit also came, but that is rather less exciting.
| + | |
| - | | + | |
| - | The sequencing results from our amino acid biobricks came back, and it looks like only MetA was successful. It should be noted that the melting temperatures of the Vf2 and the VR primers are 60°, for cPCR validation of biobricks.
| + | |
| - | | + | |
| - | Several new combinations of fluorescent genes, antibiotic resistances, and Keio strains were made. We used the wrong ones last weekend, but now, we are going to test TyrA- +GFP, TrpA- +RFP, MetA- +YFP, and also wildtype with the fluorescent genes. Recently, we switched backbones for the 13M RFP to Psb1C3, for we do not know the provenance of our current psb1C3 with rfp (we suspect it might be on the lac operon, as per the registry guidelines), and we also put the GFP on the psb1C3 instead of a kanamycin resistant plasmid, because we were transforming into kanamycin resistant auxotrophs.
| + | |
| - | | + | |
| - | Just for qualitative fun, we are trying to grow the ArgC- and the ArgE- together in M9 to see if anything results, with negative controls for both. We also tried growing a wildtype with a TrpB auxotroph with rfp to look for qualitative red fluorescence. The fluorescence calibration experiment must be done before we can do these sorts of things quantitatively.
| + | |
| - | | + | |
| - | We miniprepped 4/6 of the biobricks that we ordered from the registry. We intend on sequencing them in the near future. We, lacking LB amp plates at the time, plated one on an M9 plate, but we now think that the strain the registry sent us is auxotrophic for something. We replated on LB amp after making new plates. The last one that we ordered did not arrive.
| + | |
| - | | + | |
| - | Joe made M9 plates with and without 1 mM arginine, and it was found that the two arginine auxotrophs grew as expected.
| + | |
| - | | + | |
| - | We bought a fridge for our new office, but have yet to fill it. If anyone with extra snacks reads this and wants to help, our fridge is currently underfilled.
| + | |
| - | | + | |
| - | Overnight cultures have been set up to make competent cells out of DH5a, K12, and IGTS8.
| + | |
| - | | + | |
| - | Some new polymerase system has found its way into our freezer, and nobody seems to know its provenance. Since we do not share the freezer, we assume some kindly benefactor gifted it to us.
| + | |
| - | | + | |
| - | -Jacob
| + | |
| - | | + | |
| - | ==July 17==
| + | |
| - | | + | |
| - | Today, we made dh5a and k12 competent cells. We also ran a cPCR on our biobrick colonies that turned out to not be biobricks.
| + | |
| - | | + | |
| - | As said yesterday, we grew the two arg auxotrophs together, and they grew substantially more (~0.15 OD600) than the negative control. The wt grew to an OD of about 1. We are growing them for another night to see if the OD of the mix increases at all.
| + | |
| - | | + | |
| - | Since sequencing confirmed a MetA biobrick, we transformed K12 cells with the remaining stock of our plasmid, so we could amplify it.
| + | |
| - | | + | |
| - | All relevant transformed strains from yesterday grew well on the plates. We are now ready to properly do the fluorescence calibration experiment, but due to time constraints, we are going to do it on Thursday.
| + | |
| - | | + | |
| - | -Jacob
| + | |
| - | | + | |
| - | [[File:Pseudomonas Crystals.jpg|900x1000px]]
| + | |
| - | | + | |
| - | ==July 19==
| + | |
| - | | + | |
| - | We tested the growth rate of our 3 main auxotrophs in various (log scale from 100 pM to 10 mM) concentrations of the amino acids to do monod kinetics. We also miniprepped our metA biobrick (so we have enough to send to the registry and use for experiments), new potential candidates for other biobricks (tyrA and ArgC), our construct which is identical to 13M on plate 3, except on a different plasmid, and a biobrick that we received from the registry. Sequencing to follow.
| + | |
| - | | + | |
| - | Joe has, to the best of my knowledge, identified potential biobrick colonies for the DszB,C, and D. Again, sequencing to follow.
| + | |
| - | | + | |
| - | We have, in the past few days, designed a plasmid that will aid in the testing of biobricks. The primers are to be designed and ordered today, if all goes according to plan. The primers that we tried to order through the lab a while ago spent some time in bureaucratic limbo, but I think they have been ordered now. Again, no one appears completely sure of what has been ordered, and one of our chief contacts on the subject has apparently gone on vacation.
| + | |
| - | | + | |
| - | It appears that Joe and Grace were attempting the fluorescence calibration experiment, but things apparently were going wrong, and we have not yet analyzed the data completely.
| + | |
| - | | + | |
| - | Concerning the kill switch plasmid parts, we want to take the psb1C3 plasmid, add a constitutively expressed LacI gene, and a lac promoter and rbs immediately before the cut sites. This way, a toxic protein can be well controlled. In fact, while we are designing this for toxic proteins, it could have uses in general protein production. Previous attempts for using the lac promoter on a high copy plasmid have failed leaky expression) due to the lack of the LacI protein produced in the genome. This new plasmid design remedies this, and will be accomplished by Gibson assembly.
| + | |
| - | | + | |
| - | -Jacob
| + | |
| - | | + | |
| - | ==July 20==
| + | |
| - | | + | |
| - | Obtained the data from the 3 main auxotrophs growth rate dependences of amino acid concentration.
| + | |
| - | | + | |
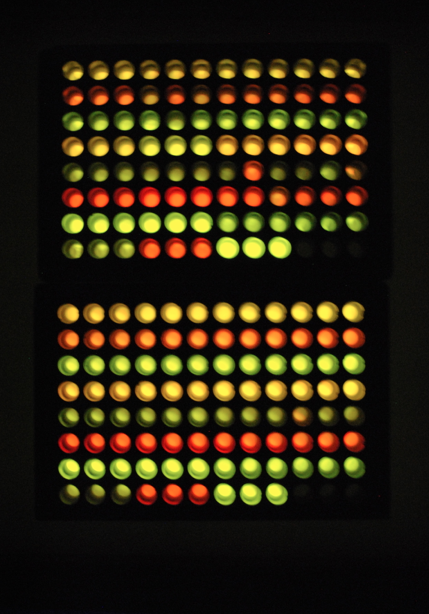
| - | Obtained the reading of fluorescence-population correlation plate.
| + | |
| - | | + | |
| - | -Ruichen
| + | |
| - | | + | |
| - | ==July 22==
| + | |
| - | | + | |
| - | Joe did 5 more fluorescence-population correlation plate, though found out that the yfp is not expressing correctly.
| + | |
| - | | + | |
| - | -Ruichen
| + | |
| - | | + | |
| - | == July 23==
| + | |
| - | | + | |
| - | Analyzed the data, and found out that the growth rate and the amino acid (Tyr) concentration has a correlation as follows:
| + | |
| - | | + | |
| - | [[File:growth-rate at different try concentration_british_columbia_2012.png|900x1000px]]
| + | |
| - | *Figure 4. cell growth-rate at different Tyr concentrations
| + | |
| - | | + | |
| - | [[File:growth-rate at different tyr concentration log scale_british_columbia_2012.png|900x1000px]]
| + | |
| - | | + | |
| - | *Figure 5. Log scale of cell growth-rate at different Tyr concentrations
| + | |
| - | | + | |
| - | It was found that the cells depleted the nutrient quickly at low Tyr concentration, and for future experiments it is suggested to use lower initial cell concentrations to extend the time of its exponential growth phase. Also, the wild type has almost the exact growth rate as the autotroph when the the Try concentration is exceptionally high (0.01 M).
| + | |
| - | | + | |
| - | | + | |
| - | -Ruichen
| + | |
| - | | + | |
| - | ==July 26==
| + | |
| - | | + | |
| - | The growth rate and the amino acids (Met and Trp) concentration has a correlation as follows:
| + | |
| - | | + | |
| - | [[File:growth-rate at different met concentration_british_columbia_2012.png|900x1000px]]
| + | |
| - | | + | |
| - | *Figure 6. cell growth-rate at different Met concentrations
| + | |
| - | | + | |
| - | [[File:growth-rate at different Met concentration log scale_british_columbia_2012.png|900x1000px]]
| + | |
| - | | + | |
| - | *Figure 7. Log scale of cell growth-rate at different Met concentrations
| + | |
| - | | + | |
| - | [[File:growth-rate at different trp concentration_british_columbia_2012.png|900x1000px]]
| + | |
| - | | + | |
| - | *Figure 8. cell growth-rate at different Trp concentrations
| + | |
| - | | + | |
| - | [[File:growth-rate at different trp concentration log scale_british_columbia_2012.png|900x1000px]]
| + | |
| - | | + | |
| - | *Figure 9. Log scale of cell growth-rate at different Trp concentrations
| + | |
| - | | + | |
| - | | + | |
| - | -Ruichen
| + | |
| - | | + | |
| - | ==August 2==
| + | |
| - | | + | |
| - | *Field trip to Chevron Burnaby site!
| + | |
| | | | |
| | [[file: refinery visit UBC 2012 1.png|900x1000px]] | | [[file: refinery visit UBC 2012 1.png|900x1000px]] |
| Line 272: |
Line 42: |
| | | | |
| | [[file: refinery visit UBC 2012 7.png|900x1000px]] | | [[file: refinery visit UBC 2012 7.png|900x1000px]] |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - | Ruichen
| |
| - |
| |
| - | ==August 3rd==
| |
| - |
| |
| - | Started to work on the data obtained on July 28th plate readings.
| |
| - |
| |
| - | Eventually arrived at the following graphs of growth rates observations, when n=3
| |
| - |
| |
| - | [[file: GrowthRatesAA_university_of_british_columbia.png|900x1000px]]
| |
| - |
| |
| - | [[file: GrowthRatesAA_LOG_university_of_british_columbia.png|900x1000px]]
| |
| - |
| |
| - | From these graphs, the maximum growth rate, and Ks values that are used in Monod Kinetics can be determined.
| |
| - |
| |
| - |
| |
| - | Ruichen
| |
| - |
| |
| - | ==August 9==
| |
| - |
| |
| - | Having trouble uploading the refinery trip pictures because the files exceed the 2MB limit! Any tips on shrinking file sizes would be much appreciated!
| |
| - |
| |
| - | - [[User:Grace.yi|grace.yi]]
| |
| - |
| |
| - | ==August 12==
| |
| - | A large set of primers arrived, which gave us a lot to do.
| |
| - |
| |
| - | With a set of the primers that arrived, we were able to PCR amplify certain parts that will be able to be Gibson assembled. On the plasmid that has RFP (a modified BBa_K093012, placed on the Psb1C3), we wanted to put arabinose inducible MetA or arabinose inducible TyrA. However, one of the primers necessary to amplify the plasmid itself, which would be necessary to incorporate the proper homologous sequence for Gibson assembly, did not arrive. Additionally, the PCR to amplify the MetA gene did not work the first time, but has been repeated. The arabinose promoter was successfully PCR'd out of the E. coli genome for both cases.
| |
| - | For the plasmid with YFP, the BBa_I13973, we wanted to put rhamnose inducible TrpA or TyrA after the YFP gene. We received all the primers necessary for this, but the PCR for the plasmid backbone gave several bands and was repeated at a higher annealing temperature. The results are not yet known. The rhamnose promoter was successfully PCR'd out of the genome, as was the TrpA gene. However, we lacked one of the promoters for the TyrA gene. Since there were several bands for the YFP plasmid, we did not do a Gibson assembly. Additionally, it is possible that the primers were designed for the pSB1C3 rather than the pSB1A2, but that should have little effect due to the similarities on the ends of the two plasmids. A simple digest and ligation should be able to move the part from the one plasmid to the other.
| |
| - | We also wanted to put a IPTG inducible (BBa_K091111, LacIQ) metA gene after a GFP. The PCR for the MetA gene and the GFP plasmid were successful (although the GFP plasmid appeared as a fairly weak band on the gel), but the PCR for the LacIQ promoter failed. The initial DNA for this PCR was added directly from the parts distribution kit, and as such the composition and concentration of exactly what was added was unknown. The PCR was repeated as a cPCR on the genome, so as to at least get a usable part with the right overhang.
| |
| - |
| |
| - | The final primers for the gibson assembly of the kill switch primer also arrived. There are four parts to this plasmid: the plasmid backbone, the lacI promoter, the constitutively expressed lacI gene, and the constitutively expressed RFP for easy selection. The plasmid backbone, the promoter, and the constitutively expressed RFP PCR products has been ready for several weeks, but we lacked the primers for the constituvely expressed lacI gene until now. To generate the DNA for the constitutively expressed lacI gene, we took BBa_K081005 (const. promoter+rbs) cut with only SpeI and BBa_I732100 (lacI) cut with only XbaI, and ligated the two together, and then did a PCR on the ligation. It worked beautifully.
| |
| - |
| |
| - | The primers also arrived to isolate the VMA Sce-I intein, and Alina provided yeast genomic DNA. The PCR was done successfully. However, upon further reflection, it appears that we forgot to DpnI digest the original template for the plasmid backbone on which we were going to add this part, and we actually did not have any product for it. Another PCR needs to be done, and DpnI digests will be done before running it on a gel.
| |
| - |
| |
| - | A previous potential part, ArgE, was restriction confirmed, and will be sent for sequencing at the soonest convenience.
| |
| - |
| |
| - | The PCR for yddg was finally a success. The part has been restriction digested, along with the pSB1C3 plasmid, and a ligation will follow.
| |
| - |
| |
| - | We finally received the DszA forward primer, and did a restriction digest on it as well. It will be ligated as well in the near future. It has several Pst1 sites, which limits some of the reactions we can do with it, but we can still place things before it by cutting with EcoRI and/or XbaI.
| |
| - |
| |
| - | In an effort to see how much amino acid each type of cell in the co-culture will produce, we have been harvesting supernatants of our normal cultures as per the protocol in Kerner et al. Once we have supernatants of the cultures at various ODs, we are going to grow the auxotrophs in this supernatant, and then look at the growth rate and final OD. The list of which supernatants we have can be found in the lab.
| |
| - |
| |
| - | Joe has found inconsistent results with his fluorescent proteins on the various promoters, and we have plans to standardize the promoter and rbs for the fluorescent proteins that will be used. Additionally, there is some overlap between YFP and GFP that is proving difficult to account for, so we are going to try to switch one to either BFP or CFP.
| |
| - |
| |
| - | We also wanted to grow three of the consortia together to see what sort of final OD they reach, and have been monitoring it sporadically for the past 24 hours.
| |
| - |
| |
| - | -Jacob
| |
| - |
| |
| - | ==August 13==
| |
| - |
| |
| - | Having harvested the supernatant of the various cultures at various ODs, we filter sterilized it with a 0.2 uM filter to remove any cells that may have still remained. Now that we have the sterilized supernatant, we are going to follow the protocol in Kerner et al 2012, and add 1/5 5X M9 media, and grow different auxotrophic cultures, noting the OD and growth rate. From this, we will try to calculate, or at least approximate, the amino acid export rate of the cells.
| |
| - |
| |
| - | There were colonies on the MinC Intein plate, the ArgE BB plate, and the GFP construct LB plate, but nothing on the GFP construct M9 plate. This may have been because there was no lactose or IPTG to induce the metA gene. Interestingly, there was a green fluorescent patch, but it was rather weak and had no distinct colonies. We plan to pick several colonies from the LB plate and try growing them in M9 culture with and without IPTG overnight. We also plan to do a cPCR on the MinC plate using the intein primers. There were no colonies on the DszA or yddg plate. We have had no luck with electroporating cells with ligation mixtures, although we have had some success with heat shock competent cells. We think there may be something in the ligation mixture that is interfering with the electroporation, and could probably be fixed by purifying the ligation mix.
| |
| - |
| |
| - | The data that we collected regarding the growth curves of the auxotrophs and the mixture are very different from what the plate reader previously showed. We think this may be because of a lack of correction for path length by the plate reader, but aren't entirely sure.
| |
| - |
| |
| - | We also did a PCR on the TyrA BB product, the previous failed YFP plasmid, and several other previously failed PCRs.
| |
| - |
| |
| - | -Jacob
| |
| - |
| |
| - | ==August 15==
| |
| - |
| |
| - | After two days of shaking at 37° in 1 mM IPTG, the supposed IPTG inducible metA constructs in the metA auxotrophs were unable to grow.
| |
| - |
| |
| - | The gels of the PCR products that we have been getting lately have not been working properly. We think that there is a problem with the buffer due to how many times it has been reusued, or possibly by the new TBE that was recently made.
| |
| - |
| |
| - | -Jacob
| |
| - |
| |
| - | ==August 16==
| |
| - |
| |
| - | The supposed metA constructs still appears unable to complement a metA knockout. It has been suggested that the concentration of metA may have been too high, and this would have led to a severe detriment to the cell, and it has also been suggested that the IPTG, being a rather old stock, was no longer good. Another step that we are going to take is to see if the expression of the construct can be seen on an sds page under induction.
| |
| - |
| |
| - | Despite, or perhaps because of this failure, we are moving on, trying to make new constructs that will be used for tunability. The YFP has yet to give us a solid band, so we are trying to move it into the psb1c3 plasmid in case there are some plasmid specific sequences that were giving us poor results. The ligation and transformation has been done, and we are waiting for colonies. The PCR products necessary for the gibson assembly of the GFP-IPTG-TrpA gene were also all available, and that gibson assembly has been done. However, due to the failure of several gels, there was very little product left, and PCR purification would likely result in unusuable quantities, so we tried a Gibson assembly with the straight PCR product, and we will see if it works.
| |
| - |
| |
| - | All of the parts necessary for the assembly of the kill switch plasmid have been successfully PCR'd, but several of the parts fall victim to the same caveat as that of the previously discussed products. That being said, we tried it anyways,and will check for colonies tomorrow.
| |
| - |
| |
| - | The TBE that we were using for the gels has not been working for anyone in the lab, although it has been suggested that that may simply because people don't change their buffers. The TAE gels work fine.
| |
| - |
| |
| - | Another PCR was done to generate some of the other parts required for assembly of other parts. The new forward primer for the arabinose promoter does not seem to be working, although the old one worked well. We recently ran out of phusion polymerase, and have resorted to using an eclectic mix of collected polymerases in our PCR box.
| |
| - |
| |
| - | A cPCR of the colonies with the MinC protein with the Sce VME intein showed that the gene for the intein was present in the cells that were supposed to carry it! A small victory, perhaps, but it shows that the Gibson assembly works, contrary to out experiences with the positive control. A site directed mutagenesis must be performed on the gene to convert an internal lysine into a phenylalanine, which has been shown to make the intein temperature sensitive. The primers for this reaction have been eluted, but we are still waiting for a plasmid stock of the minC-intein plasmid. By the way, the MinC protein was chosen because it can be used as a kill switch and has the necessary sequence (G-C) that the intein uses for self excision.
| |
| - |
| |
| - | There are two distinct bands on the RFP PCR, one at the correct length, the other much lower. Seems like a perfect chance for gel extraction. Several other products were also generated today, but there are still two essential parts, that of YFP and that of the new arabinose promoter, which have not yet been successfully PCR'd.
| |
| - |
| |
| - | The cPCR on the DszA colonies gave no result. This may have been due to an unoptimized Taq protocol,and might be worth repeating.
| |
| - |
| |
| - | -Jacob
| |
| - |
| |
| - | ==August 17==
| |
| - |
| |
| - | The Gibson assembled parts using unpurified PCR products did not work. I think it was because of the extra salts, etc that would be present in the PCR mix. As a note, make sure to somehow purify your PCR product before doing a Gibson assembly!
| |
| - |
| |
| - | There were many colonies on the 6I ligation heat shock (but not electroshock) plate. However, there were of insufficient size to work with, so they were left to grow for another day (or 2, considering how the power will be out on the 18th).
| |
| - |
| |
| - | The plans for the metA construct induction were further discussed. Overnight colonies will be made of the construct and a negative control, then they will be diluted in 1 in 100 LB, allowed to grow to 0.4 OD, induced with 0.1 mM new IPTG, and harvested at a certain OD following this, then run on an SDS PAGE. This will test to see if the IPTG really would stimulate the production of the metA gene. The negative controls were not in place yet. Four potential constructs have been grown up overnight, and are labeled 1-4 on tape in the right section of the left shaker. Is there some label on the metA protein that we could use to do a Western?
| |
| - |
| |
| - | The data from the supernatant growth experiment was partially analyzed. It appears that 14 hours was not sufficient to reach a final OD in some cases, but there was growth in many of the wells. It was also suggested that we could just test the relevant amino acid concentration using HPLC. One thing that I was wary about concerning this is that the HPLC would only pick up pure amino acid, and if there was, for instance, a short protein fragment, it could be used for growth, but not show up on the HPLC. Any thoughts?
| |
| - |
| |
| - | The cultures for the MinteinC (As it shall henceforth be known) grew well, but were not miniprepped. Once they are miniprepped, then site directed mutagenesis must be done. The cultures of the potential yddg biobricks grew well, and will be confirmed either by PCR of the plasmid using VR and VF2, or by restriction digest.
| |
| - |
| |
| - | From our previous experiments concerning the consortia syntrophy, we saw that three cultures inoculated straight from LB into minimal M9 led to a mixed culture OD that reached about 1.3, similar to 0.1 mM (saturating) Met for metA-, and 0.1 mM Trp for TrpA-. We suspect that this is due to syntrophy, but have yet to do the relevant tests to see if 1 in 100 LB actually does supply significant amounts of amino acids.
| |
| - |
| |
| - | I will be going on vacation for the next couple of weeks, and will return in September.
| |
| - |
| |
| - | -Jacob
| |
| - |
| |
| - | ---------------------------------------------------------------------------------------
| |
| - | the following is only for '''iGEM Life''' section, as i failed to find the sub-group category working.
| |
| - |
| |
| - |
| |
| - | Working environment
| |
| - |
| |
| | | | |
| | [[File:Ourbuilding_british_columbia_2012.png|center|300x500px]] | | [[File:Ourbuilding_british_columbia_2012.png|center|300x500px]] |
| Line 395: |
Line 49: |
| | [[File:LabCondition_british_columbia_2012.png|center|300x500px]] | | [[File:LabCondition_british_columbia_2012.png|center|300x500px]] |
| | | | |
| - | ==July 31st -- Aug 1st==
| + | |
| | + | '''July 31 - Aug 1''' |
| | | | |
| | Group Social-- Dural Birthday celebrations | | Group Social-- Dural Birthday celebrations |
| | | | |
| | [[File:BirthdayDinner_british_columbia_2012.png|center|500x700px]] | | [[File:BirthdayDinner_british_columbia_2012.png|center|500x700px]] |
| - |
| |
| | | | |
| | [[File:BirthdayGranvilleIsland_british_columbia_2012.png|center|500x700px]] | | [[File:BirthdayGranvilleIsland_british_columbia_2012.png|center|500x700px]] |
| | | | |
| | [[File:BirthdayGranvilleIsland2_british_columbia_2012.png|center|500x700px]] | | [[File:BirthdayGranvilleIsland2_british_columbia_2012.png|center|500x700px]] |
| - |
| |
| | | | |
| | [[File:Modelling_Meeting_british_columbia_2012.png|center|500x700px]] | | [[File:Modelling_Meeting_british_columbia_2012.png|center|500x700px]] |
| | | | |
| - | ==Sept 20th==
| + | '''Sept 20''' |
| | | | |
| | [[File:Jacobsolo_british_columbia_2012.png|center]] | | [[File:Jacobsolo_british_columbia_2012.png|center]] |
| | | | |
| | | | |
| | + | '''Sept 15-16''' |
| | | | |
| - | | + | Traveling to Edmonton for aGEM! |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | ==Sept 15==
| + | |
| - | | + | |
| - | | + | |
| - | '''Traveling to Edmonton for aGEM!! '''
| + | |
| - | | + | |
| | | | |
| | [[File:AirportMeetUp_british_columbia_2012.png|centre|400x500px]] | | [[File:AirportMeetUp_british_columbia_2012.png|centre|400x500px]] |
| | | | |
| | + | Team Dinner -at Vancouver International Airport(YVR) |
| | | | |
| - | '''Team Dinner -at Vancouver International Airport(YVR)'''
| + | [[File:Team_aGEM_airport_Dinner_british_columbia_2012.png|centre|400x500px]] |
| | | | |
| - |
| |
| - | [[File:Team_aGEM_airport_Dinner_british_columbia_2012.png|centre|400x500px]]
| |
| | [[File:Team_aGEM_airport_Dinner_2_british_columbia_2012.png|centre|400x500px]] | | [[File:Team_aGEM_airport_Dinner_2_british_columbia_2012.png|centre|400x500px]] |
| - |
| |
| | | | |
| | [[File:Team_aGEM_Mikestressedout_british_columbia_2012.png|centre|400x500px]] | | [[File:Team_aGEM_Mikestressedout_british_columbia_2012.png|centre|400x500px]] |
| Line 441: |
Line 84: |
| | | | |
| | [[File:Team_aGEM_presentationpreparation_british_columbia_2012.png|centre|400x500px]] | | [[File:Team_aGEM_presentationpreparation_british_columbia_2012.png|centre|400x500px]] |
| - |
| |
| - | ==Sept 16==
| |
| | | | |
| | [[File:Team_aGEM_signingin_british_columbia_2012.png|centre|400x500px]] | | [[File:Team_aGEM_signingin_british_columbia_2012.png|centre|400x500px]] |
| Line 452: |
Line 93: |
| | | | |
| | | | |
| - |
| |
| - | ------------------------------------------------------------------------
| |
| - | the following is for '''wet lab'''
| |
| | | | |
| | [[File:Joe1Team_british_columbia_2012.png|centre|400x500px]] | | [[File:Joe1Team_british_columbia_2012.png|centre|400x500px]] |
| Line 461: |
Line 99: |
| | | | |
| | [[File:Jacob1Team_british_columbia_2012.png|centre|400x500px]] | | [[File:Jacob1Team_british_columbia_2012.png|centre|400x500px]] |
| | + | |
| | [[File:Jacob2Team_british_columbia_2012.png|centre|400x500px]] | | [[File:Jacob2Team_british_columbia_2012.png|centre|400x500px]] |
| | + | |
| | [[File:Jacob3Team_british_columbia_2012.png|centre|400x500px]] | | [[File:Jacob3Team_british_columbia_2012.png|centre|400x500px]] |
| | + | |
| | [[File:Ruichen_Xue_1_Team_british_columbia_2012.png|centre|400x500px]] | | [[File:Ruichen_Xue_1_Team_british_columbia_2012.png|centre|400x500px]] |
| | + | |
| | [[File:Ruichen_Xue_2_Team_british_columbia_2012.png|centre|400x500px]] | | [[File:Ruichen_Xue_2_Team_british_columbia_2012.png|centre|400x500px]] |
| | + | |
| | [[File:Mehul1_Team_british_columbia_2012.png|centre|400x500px]] | | [[File:Mehul1_Team_british_columbia_2012.png|centre|400x500px]] |
| | | | |
| | [[File:Joe_4degroom_Team_british_columbia_2012.png|centre|400x500px]] | | [[File:Joe_4degroom_Team_british_columbia_2012.png|centre|400x500px]] |
| | + | |
| | [[File:4degroom_Team_british_columbia_2012.png|centre|400x500px]] | | [[File:4degroom_Team_british_columbia_2012.png|centre|400x500px]] |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| | | | |
| | [[File:Plate_reader_Team_british_columbia_2012.png|centre|400x500px]] | | [[File:Plate_reader_Team_british_columbia_2012.png|centre|400x500px]] |
| Line 479: |
Line 119: |
| | | | |
| | [[File:ConsoriaCells_british_columbia_2012.png|centre|400x500px]] | | [[File:ConsoriaCells_british_columbia_2012.png|centre|400x500px]] |
| | + | |
| | [[File:ConsoriaCells2_british_columbia_2012.png|centre|400x500px]] | | [[File:ConsoriaCells2_british_columbia_2012.png|centre|400x500px]] |
| | | | |
| | + | [[File:ConsoriaPlates1_british_columbia_2012.png|centre|400x500px]] |
| | | | |
| - | [[File:ConsoriaPlates1_british_columbia_2012.png|centre|400x500px]]
| |
| | [[File:ConsoriaPlates2_british_columbia_2012.png|centre|400x500px]] | | [[File:ConsoriaPlates2_british_columbia_2012.png|centre|400x500px]] |
| | + | |
| | [[File:ConsoriaPlates3_british_columbia_2012.png|centre|400x500px]] | | [[File:ConsoriaPlates3_british_columbia_2012.png|centre|400x500px]] |
| | + | |
| | [[File:ConsoriaPlates4_british_columbia_2012.png|centre|400x500px]] | | [[File:ConsoriaPlates4_british_columbia_2012.png|centre|400x500px]] |
| - |
| |
| | | | |
| | [[File:ConsumptionRateMeasurement_Team_british_columbia_2012.png|centre|400x500px]] | | [[File:ConsumptionRateMeasurement_Team_british_columbia_2012.png|centre|400x500px]] |
| - |
| |
| | | | |
| | [[File:RunningHugeGel_Team_british_columbia_2012.png|centre|400x500px]] | | [[File:RunningHugeGel_Team_british_columbia_2012.png|centre|400x500px]] |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - | - [[User:Ruichen|Ruichen]]
| |

 "
"