Team:Cambridge/Project/Results
From 2012.igem.org
(→Ratiometrica) |
|||
| Line 199: | Line 199: | ||
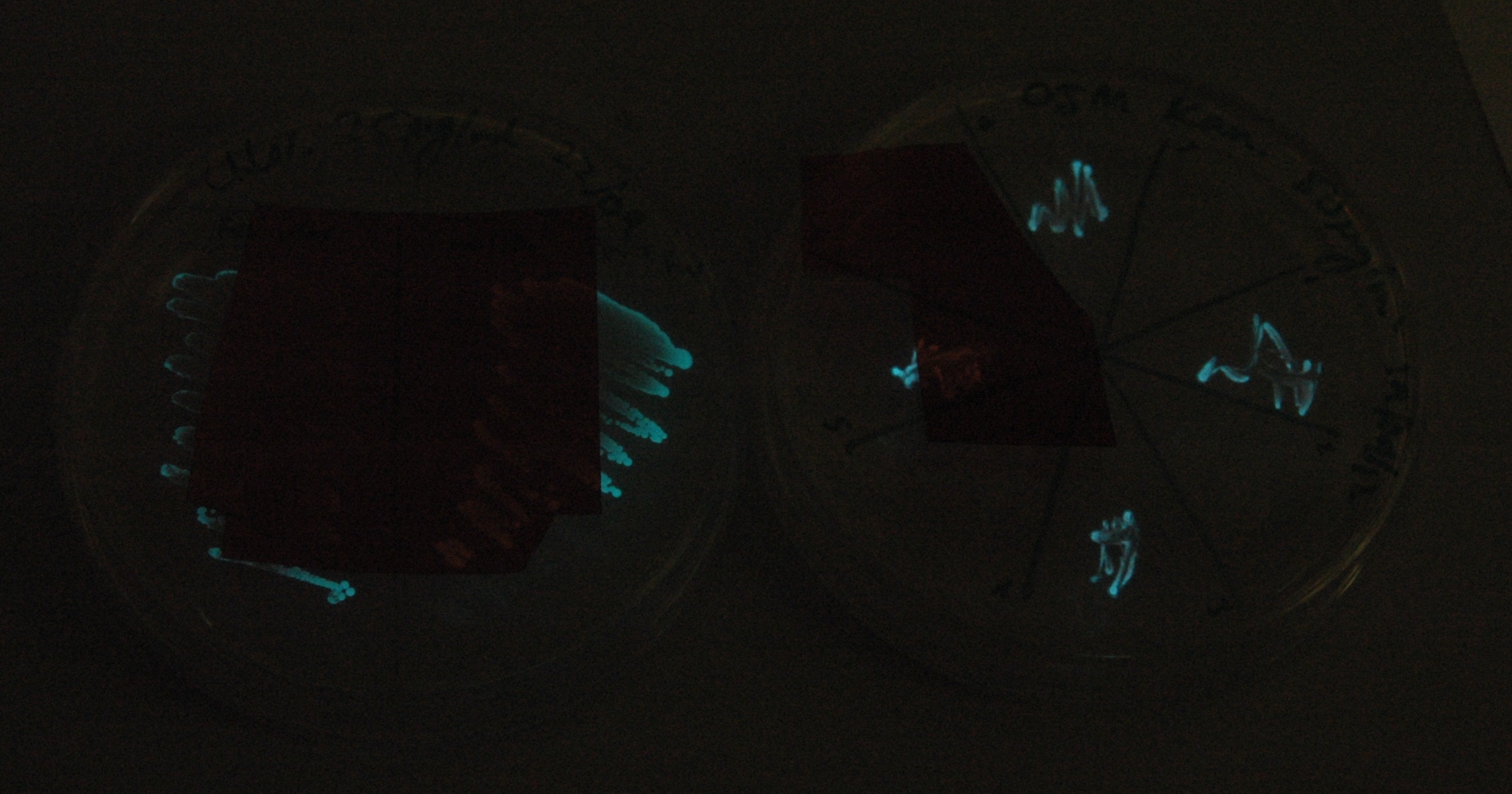
[[File:Lux_colour_change_image_1_small.jpg|450px|center|thumb| 30 second exposure, K325909 on the left, our construct on the right.]] | [[File:Lux_colour_change_image_1_small.jpg|450px|center|thumb| 30 second exposure, K325909 on the left, our construct on the right.]] | ||
| - | [[File:Lux_colour_change_image_2.jpg|450px|center|thumb| As above, but with the filters shifted so that the | + | [[File:Lux_colour_change_image_2.jpg|450px|center|thumb| As above, but with the filters shifted so that the previously filtered areas are visible for comparison.]] |
| - | This was done at quite a late stage and in a slightly impromptu fashion. The photographs | + | This was done at quite a late stage and in a slightly impromptu fashion. The photographs above directly compare the normal lux biobrick (BBa_K325909) on the left, and our construct on the right, with the same filter. There does seem to be a difference in the quantity of light coming through the filter. However it could be due to colony density (although they seem to be of similar intensities without the filter). Interesting though these results are, they are not quantitative or well-controlled enough to constitute confirmation that the luxA-mOr fusion is behaving as expected, rather they are an indication that it may be. We do not currently have access to a scanning luminometer to fully characterise the emission spectrum, however we hope to be able to present this data at the jamboree. |
===Instrumentation (Biologger)=== | ===Instrumentation (Biologger)=== | ||
Revision as of 00:00, 27 September 2012


Contents |
Results
Set out below are the developments the team has made over the summer, in tackling our aim and objectives.
RiboSense
Ratiometrica
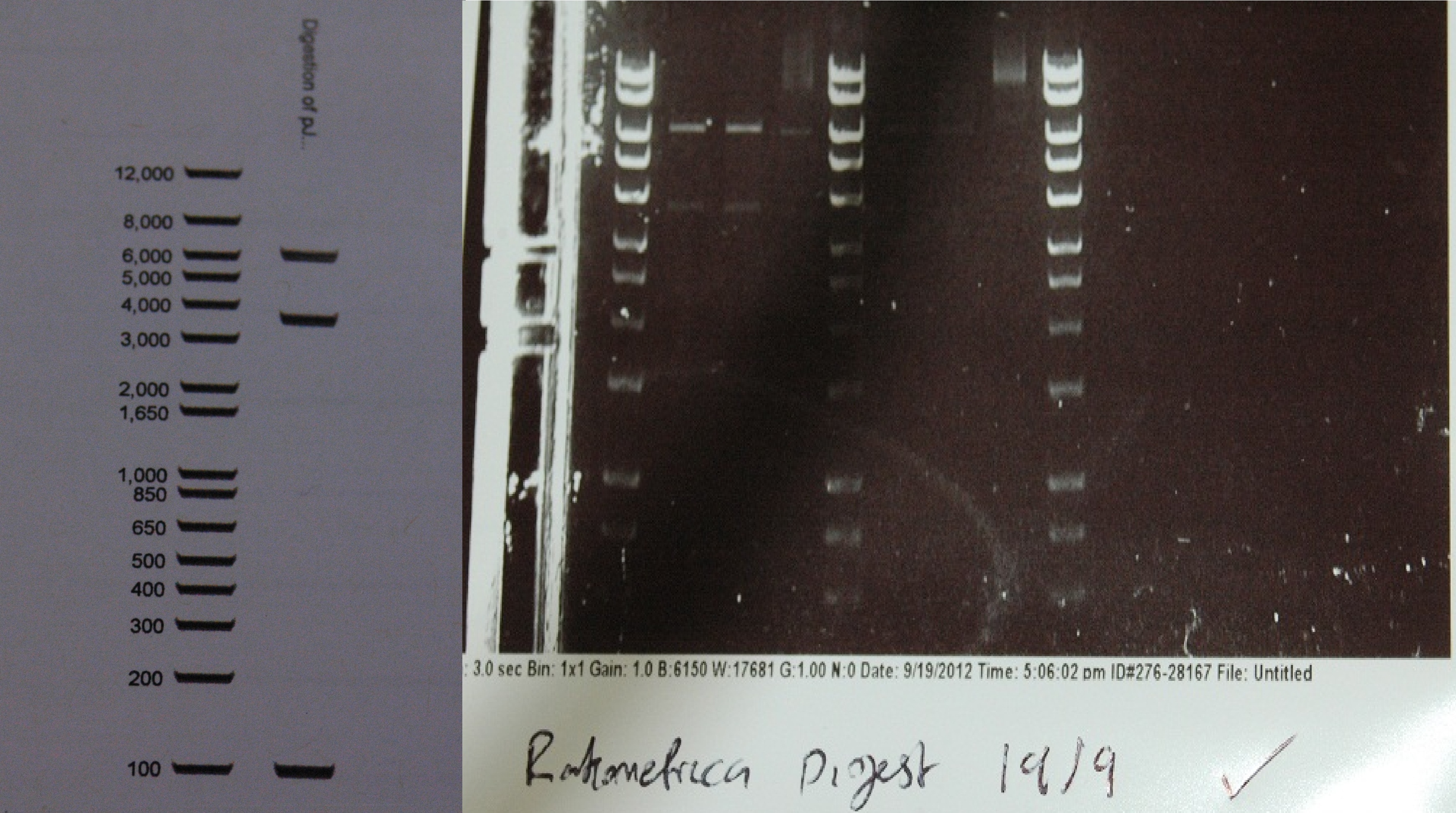
After a lot of technical difficulties, we were able to assemble our fluorescent construct using Gibson assembly. The photo below shows the predicted and obtained digest pattern using HINDIII.
The construct is also sequenced from the beginning and middle (as it is a long part) by Source Bioscience, below is the alignment data from the sequencing (using ClustalW2):
CLUSTAL 2.1 multiple sequence alignment
seq ---------------------------NNNNNNNNNNNAANNNNNNNA-- 21
pJS150_seq_-_Ratiometrica AGCCCAGTCCAGACTATTCGGCACTGAAATTATGGGTGAAGTGGTCAAGA 1050
.. ......**..... *
seq ---------------------------------------NNNNNNNTNTN 32
pJS150_seq_-_Ratiometrica CCTCACTAGGCACCTTAAAAATAGCGCACCCTGAAGAAGATTTATTTGAG 1100
... ..*.:.
seq GTN--CNNT----------------------------------------- 39
pJS150_seq_-_Ratiometrica GTAGCCCTTGCCTACCTAGCTTCCAAGAAAGATATCCTAACAGCACAAGA 1150
** * .*
seq -----------TTNNNNTCTN-------------CTAANTNTNAGNGCTC 65
pJS150_seq_-_Ratiometrica GCGGAAAGATGTTTTGTTCTACATCCAGAACAACCTAATTGTGAGCGCTC 1200
**....*** ****.*.*.** ****
seq NNAATTTTTTGNNNAATTNNTNANNNTTTATCTACN-GGTGTGTCAT-AT 113
pJS150_seq_-_Ratiometrica ACAATTTTTTGCAAAAAGTTGTTGACTTTATCTACAAGGTGTGGCATAAT 1250
********* **: .. .:. ********* ****** *** **
seq GTNTGGAANNNCNANNAGCTCACAATTAANGGATGAATTCN-AATGGTGA 162
pJS150_seq_-_Ratiometrica GTGTGGAATTGTGAGCGGCTCACAATTAAAGGAGGAATTCAAAATGGTGA 1300
**.*****... .*. .************ *** ****** ********
seq GCAAGGGCGANGAGCTGTTCACCGGGGTGGNGCCCATCCTGGTCGAGCTG 212
pJS150_seq_-_Ratiometrica GCAAGGGCGAGGAGCTGTTCACCGGGGTGGTGCCCATCCTGGTCGAGCTG 1350
**********.*******************.*******************
seq GACGGCGACGNGANCGGCNACAAGTTCATCGTGTCCTNCGAGGGCGAGGG 262
pJS150_seq_-_Ratiometrica GACGGCGACGTGAACGGCCACAAGTTCAGCGTGTCCGGCGAGGGCGAGGG 1400
**********.** **** ********* ******* .************
seq CGATGCCACCTACGGCAAGCTGACCTTGAAGTTCATCTG-ACCACCGGCA 311
pJS150_seq_-_Ratiometrica CGATGCCACCTACGGCAAGCTGACCCTGAAGTTCATCTGCACCACCGGCA 1450
************************* ************* **********
seq AGCTGCCCGTGCCCTGGCCCACCNTCGTGACCACCCTGACTTGGGGCGTG 361
pJS150_seq_-_Ratiometrica AGCTGCCCGTGCCCTGGCCCACCCTCGTGACCACCCTGACCTGGGGCGTG 1500
*********************** **************** *********
seq CANTGCTTCTNCCGCTACCCCGACCACATGAAGCANCANGACTTCTTCAN 411
pJS150_seq_-_Ratiometrica CAGTGCTTCAGCCGCTACCCCGACCACATGAAGCAGCACGACTTCTTCAA 1550
**.******:.************************.** **********
seq TTCCGCCATGCCCGAAGGCTACTTCNAGNAGCTCACCNTCTTCTTNAAGG 461
pJS150_seq_-_Ratiometrica GTCCGCCATGCCCGAAGGCTACGTCCAGGAGCGCACCATCTTCTTCAAGG 1600
********************* ** **.*** **** ******* ****
seq ACNACGGCAACTACAT--------------TNAN---------------- 481
pJS150_seq_-_Ratiometrica ACGACGGCAACTACAAGACCCGCGCCGAGGTGAAGTTCGAGGGCGACACC 1650
**.************: *.*
seq --------------------------------------------------
pJS150_seq_-_Ratiometrica CTGGTGAACCGCATCGAGCTGAAGGGCATCGACTTCAAGGAGGACGGCAA 1700
CLUSTAL 2.1 multiple sequence alignment
Rat3 -----------------------------NNNNNNNNNNNNNNNNNNTTN 21
pJS150_seq_-_Ratiometrica AGAGTCACACTGGCTCACCTTCGGGTGGGCCTTTCTGCGTTTATAAATTT 2150
... .. .... . **.
Rat3 TGNCNNN-TAATTTTATTGACAACGTCTTATTAACGTTGATATAATTTAA 70
pJS150_seq_-_Ratiometrica TGTCAAAATAATTTTATTGACAACGTCTTATTAACGTTGATATAATTTAA 2200
**.* ******************************************
Rat3 ATTTTATTTGN-NAAAATGGGCTCGTGTTGTACAATAAATGTTACTAGAG 119
pJS150_seq_-_Ratiometrica ATTTTATTTGACAAAAATGGGCTCGTGTTGTACAATAAATGTTACTAGAG 2250
********** *************************************
Rat3 AAAGGTGGTGNATACTAGATGGTGAGCAAGGGCGAGGAGCTGTTCACCGG 169
pJS150_seq_-_Ratiometrica AAAGGTGGTGAATACTAGATGGTGAGCAAGGGCGAGGAGCTGTTCACCGG 2300
********** ***************************************
Rat3 GGTGGTGCCCATCCTGGTCGAGCTGGACGGCGACGTAAACGGCCACAAGT 219
pJS150_seq_-_Ratiometrica GGTGGTGCCCATCCTGGTCGAGCTGGACGGCGACGTAAACGGCCACAAGT 2350
**************************************************
Rat3 TCAGCGTGTCCGGCGAGGGCGAGGGCGATGCCACCTACGGCAAGCTGACC 269
pJS150_seq_-_Ratiometrica TCAGCGTGTCCGGCGAGGGCGAGGGCGATGCCACCTACGGCAAGCTGACC 2400
**************************************************
Rat3 CTGAAGTTCATCTGCACCACCGGCAAGCTGCCCGTGCCCTGGCCCACCCT 319
pJS150_seq_-_Ratiometrica CTGAAGTTCATCTGCACCACCGGCAAGCTGCCCGTGCCCTGGCCCACCCT 2450
**************************************************
Rat3 CGTGACCACCTTCGGCTACGGCCTGCAATGCTTCGCCCGCTACCCCGACC 369
pJS150_seq_-_Ratiometrica CGTGACCACCTTCGGCTACGGCCTGCAATGCTTCGCCCGCTACCCCGACC 2500
**************************************************
Rat3 ACATGAAGCTGCACGACTTCTTCAAGTCCGCCATGCCCGAAGGCTACGTC 419
pJS150_seq_-_Ratiometrica ACATGAAGCTGCACGACTTCTTCAAGTCCGCCATGCCCGAAGGCTACGTC 2550
**************************************************
Rat3 CAGGAGCGCACCATCTTCTTCAAGGACGACGGCAACTACAAGACCCGCGC 469
pJS150_seq_-_Ratiometrica CAGGAGCGCACCATCTTCTTCAAGGACGACGGCAACTACAAGACCCGCGC 2600
**************************************************
Rat3 CGAGGTGAAGTTCGAGGGCGACACCCTGGTGAACCGCATCGAGCTGAAGG 519
pJS150_seq_-_Ratiometrica CGAGGTGAAGTTCGAGGGCGACACCCTGGTGAACCGCATCGAGCTGAAGG 2650
**************************************************
Rat3 GCATCGACTTCAAGGAGGACGGCAACATCCTGGGGCACAAGCTGGAGTAC 569
pJS150_seq_-_Ratiometrica GCATCGACTTCAAGGAGGACGGCAACATCCTGGGGCACAAGCTGGAGTAC 2700
**************************************************
Rat3 AACTACAACAGCCACAACGTCTATATCATGGCCGACAAGCAGAAGAACGG 619
pJS150_seq_-_Ratiometrica AACTACAACAGCCACAACGTCTATATCATGGCCGACAAGCAGAAGAACGG 2750
**************************************************
Rat3 CATCAAGGTGAACTTCAAGATCCGCCACAACATCGAGGACGGCAGCGTGC 669
pJS150_seq_-_Ratiometrica CATCAAGGTGAACTTCAAGATCCGCCACAACATCGAGGACGGCAGCGTGC 2800
**************************************************
Rat3 AGCTCGCCGACCACTACCAGCAGAACACCCCCATCGGCGACGGCCCCGTG 719
pJS150_seq_-_Ratiometrica AGCTCGCCGACCACTACCAGCAGAACACCCCCATCGGCGACGGCCCCGTG 2850
**************************************************
Rat3 CTGCTGCCCGACAACCACTACCTGAGCTACCAGTCCGCCCTGAGCAAAGA 769
pJS150_seq_-_Ratiometrica CTGCTGCCCGACAACCACTACCTGAGCTACCAGTCCGCCCTGAGCAAAGA 2900
**************************************************
Rat3 CCCCNAACGAGAAGCGCGATCACATGGTCCTGCTGGAGTTCGTGACCGCC 819
pJS150_seq_-_Ratiometrica CCCC-AACGAGAAGCGCGATCACATGGTCCTGCTGGAGTTCGTGACCGCC 2949
**** *********************************************
Rat3 GCCGGGATCACTCTCGGCATGGACGAGCTGTACAAGTAATAATGATACTA 869
pJS150_seq_-_Ratiometrica GCCGGGATCACTCTCGGCATGGACGAGCTGTACAAGTAATAATGATACTA 2999
**************************************************
Rat3 GAGCCAGGCATCAAATAAAACGAAAGGCTCAGTCGAAAGACTGGGCCTTT 919
pJS150_seq_-_Ratiometrica GAGCCAGGCATCAAATAAAACGAAAGGCTCAGTCGAAAGACTGGGCCTTT 3049
**************************************************
Rat3 CGTTTTATCTGTTGTTTGTCGGTGAACGCTCTCTACTAGTCATCATTTCC 969
pJS150_seq_-_Ratiometrica CGTTTTATCTGTTGTTTGTCGGTGAACGCTCTCTACTAGTCATCATTTCC 3099
**************************************************
Rat3 TTCCGAAAAAACGGTTGCATTTAAATCTTACATATGTAATACTTTTCAAA 1019
pJS150_seq_-_Ratiometrica TTCCGAAAAAACGGTTGCATTTAAATCTTACATATGTAATACTTT-CAAA 3148
********************************************* ****
Rat3 GACTACATTTTGTAAGATTTGATGTTTGAANNCGGGCTGAAANATCGGTA 1069
pJS150_seq_-_Ratiometrica GACTACATTT-GTAAGATTTGATGTTTGAG-TCGG-CTGAAAGATCG-TA 3194
********** ******************. .*** ******.**** **
Rat3 CGTACCCNNNNTTGTTTCNNNATNNNNCAG-CCNATNNNCTNNNNGNATA 1118
pJS150_seq_-_Ratiometrica CGTACCAATTATTGTTTCGTGATTGTTCAAGCCATAACACTGTAGGGATA 3244
******. .. *******...**....**. ** :: **.. .*.***
Rat3 NNNNNNNAAGAGNNCTTCNNCNGGNNACNANTCANNNAANNANTNAANCN 1168
pJS150_seq_-_Ratiometrica GTGG--AAAGAGTGCTTCATCTGGTTACGA-TCAATCAAATATTCAAACG 3291
.... *****..**** .*.**..**.* *** . ** .*.* ** *.
Rat3 GNNGGNNNACNNNNTNNNN---ANNNNNNANNTTNNNCGAANNN------ 1209
pJS150_seq_-_Ratiometrica GAGGGAG-ACGATTTTGATGAAACCAGTAACGTTATACGATGTCGCAGAG 3340
* .** . **. ..*.. . * .. * .** . ***:..
Rat3 ----CCNNNNNNNNTNNNNNNNNGNNNNNNNNNANNN------------- 1242
pJS150_seq_-_Ratiometrica TATGCCGGTGTCTCTTATCAGACCGTTTCCCGCGTGGTGAACCAGGCCAG 3390
**..... . *. . . .... . ....
We are submitting an RFC in the hope to propose using this construct as a new standard to characterise promoters, both constitutive and inducible, in E. coli and B. subtilis. Click here to see our draft RFC.
Our ratiometric luciferase construct arrived with full sequence coverage. Unfortunately, it was unexpectedly toxic. This hampered characterisation, as it the construct tended to be lost, and necessitated its submission to the registry in a low copy number backbone (with permission from HQ).
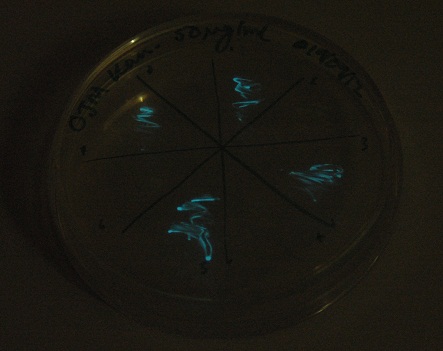
Starting from the ground up, we first showed that the part was constitutively luminescing. We also observed that the colonies were orange. This was not initially expected, however this is probably because we designed the construct with consensus RBSes, so any leaky transcription through the inducible promoter would result in enough protein to be visible. We showed that orange colour and luminescence cosegregated, implying that the entire construct is being lost, not just part of it.
Given that IPTG induction seemed unnecessary for production of mOrange, we reasoned that any spectral shift in the emission of the luciferase should be visible without induction. Our next move was to see if we could detect a difference with the filters we used for the instrumentation.
This was done at quite a late stage and in a slightly impromptu fashion. The photographs above directly compare the normal lux biobrick (BBa_K325909) on the left, and our construct on the right, with the same filter. There does seem to be a difference in the quantity of light coming through the filter. However it could be due to colony density (although they seem to be of similar intensities without the filter). Interesting though these results are, they are not quantitative or well-controlled enough to constitute confirmation that the luxA-mOr fusion is behaving as expected, rather they are an indication that it may be. We do not currently have access to a scanning luminometer to fully characterise the emission spectrum, however we hope to be able to present this data at the jamboree.
Instrumentation (Biologger)
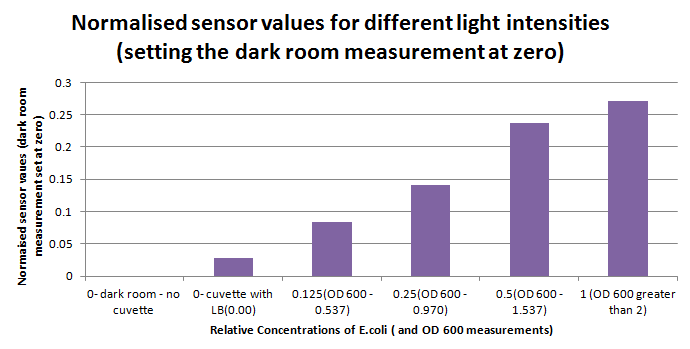
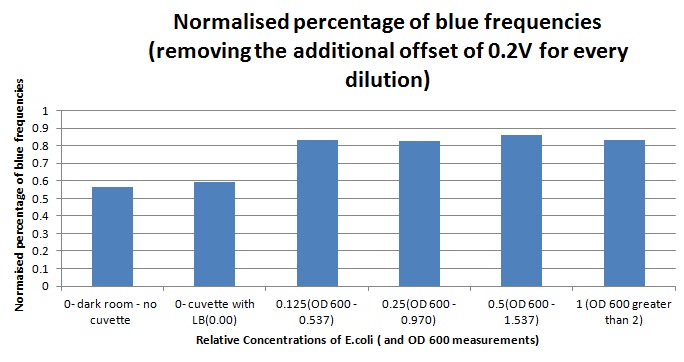
Having our instrumentation completed, as can be seen in our Instrumentation (Biologger) page, the sensitivity of the sensor placed in the right position was tested using a dilution series of luciferase-producing E.coli. 20ml Cultures were grown overnight from single colonies. The cultures were induced with 40ul of 1.5M arabinose (for a final concentration of 3mM). Cultures were left for 2 1/2 hours for full induction. Subsequently, a culture was pelleted and resuspended in 4ml LB. Doubling dilutions, of volume 2ml, were made from this concentrate, down to 1/8th concentration. 1ml of each 2ml dilution was analysed in each cuvette, which was placed in the cuvette holder we made ourselves. The result was very good. An almost linear relationship was obtained when data were normalised with the sensor value taken in the dark room (the latter set at zero) without using the cuvette holder (1-(sensor value/sensor value in absolute dark)), presenting the sensitivity of the sensor to different intensities of light. This behaviour was expected due to the changing offset affecting the luciferase spectrum curve at different light intensities. The offset, using our data, was calculated to be about 0.2V for each dilution. A second graph is shown which takes into account this offset (and removes it), thus showing the presence of blue frequencies. The result was as expected, as the presence of blue frequencies throughout the dilution series is not only detected, but also found to be approximately constant. The raw data of this investigation can be found in the Lab book.
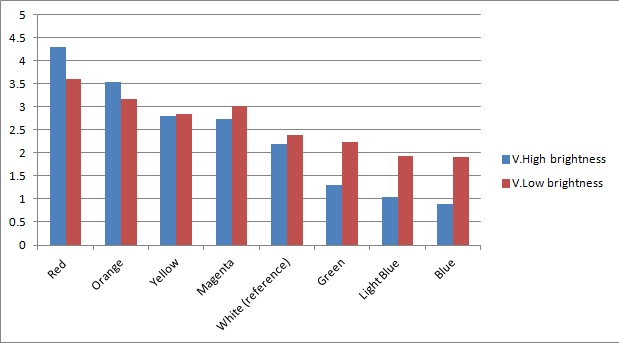
Once the sensor was tested for sensitivity, we tested that our circuitry correctly identified different frequencies (colours) of light. As can be seen below, measurements taken from orange and blue light yield values respectively above and below those from white light (our reference point). The data was taken using constant intensity of light for each case (V.High and V.Low brightness, as specified in the application). This was done with the aid of an Android phone and a specialised software application, called Color Flashlight, downloaded from the official Market.
As expected from the potential-divider design of our circuitry, orange and red frequencies caused the resistance of the LDR with the orange filter to decrease, leading to a higher voltage across the LDR with the blue filter. The opposite effect was observed with blue light. The reason that the white reference point is a bit lower than 2.5V (the expected value for a non-biased circuitry with a 5V source), is because we use resistors of total net resistance 1.67 kΩ before the blue LDR. This was done to bias the circuitry towards blue (i.e. decreasing the starting value, thus the sensor identifies always a bit more blue - this can be shown in previous graphs as well) and thus cause orange light to have a larger impact when present. This was used in an attempt to compensate for the fact that the peak at 560nm (Orange) in MOrange/luciferase fusion spectrum is lower than the one at 490 nm (Blue). Even though we did not manage to test the latter with transformed bacteria, the data collected in all the previous experiments makes us confident that the instrumentation is at least adequately functional.
As the major part of the instrumentation, the bio-electronic interface, had been made and tested, now we turned to testing the other parts of our deveoped kit. This included the mechanical chassis of the prototype, the electronic/mechatronic (sensory and motory) components, and of course the software. The overview of the finished hardware/software can be seen in our Intstrumentation (Biologger) page. Below, the videos showing our instrumentation in action can be seen.
 "
"