Team:TU Munich/Project/Limonene
From 2012.igem.org
(→Limonene) |
(→Limonene) |
||
| Line 14: | Line 14: | ||
Doesn't that make you thirsty? Then you're probably an Englishman - | Doesn't that make you thirsty? Then you're probably an Englishman - | ||
| - | + | Just think of the refreshing sensation of lemons paired with the complex richness of a <s>chilled</s> ''tepid'' brew. | |
'''Limonene''' is a cyclic terpene and a major constituent of several citrus oils. D-Limonene is used as a component of flavorings and fragrances since it has an orange/lemon-like odor. Limonene has been shown to inhibit rat mammary and other tumor development [[http://www.ncbi.nlm.nih.gov/pubmed?term=Tsuda%20limonene%202004 Tsuda et al., 2004]]. Being an excellent solvent of cholesterol, d-limonene also has been used clinically to dissolve cholesterol-containing gallstones. Because of its gastric acid neutralizing effect and its support of normal peristalsis, it has also been used for relief of heartburn [[http://www.ncbi.nlm.nih.gov/pubmed/18072821 Sun, 2007]]. | '''Limonene''' is a cyclic terpene and a major constituent of several citrus oils. D-Limonene is used as a component of flavorings and fragrances since it has an orange/lemon-like odor. Limonene has been shown to inhibit rat mammary and other tumor development [[http://www.ncbi.nlm.nih.gov/pubmed?term=Tsuda%20limonene%202004 Tsuda et al., 2004]]. Being an excellent solvent of cholesterol, d-limonene also has been used clinically to dissolve cholesterol-containing gallstones. Because of its gastric acid neutralizing effect and its support of normal peristalsis, it has also been used for relief of heartburn [[http://www.ncbi.nlm.nih.gov/pubmed/18072821 Sun, 2007]]. | ||
Revision as of 16:02, 26 September 2012



Limonene
A common saying is "If life gives you lemons - make lemonade". As of iGEM it can only be "If life gives you limonene - make beer".
The obvious thing to do, isn't it? Beer with lemonade is a very popular beverage throughout Germany, e.g. "Radler", "Alsterwasser", "Russ'n". So why not take the shortcut and skip the intermediary?
Just think of the refreshing sensation of lemons paired with the complex richness of a chilled brew.
Doesn't that make you thirsty? Then you're probably an Englishman -
Just think of the refreshing sensation of lemons paired with the complex richness of a chilled tepid brew.
Limonene is a cyclic terpene and a major constituent of several citrus oils. D-Limonene is used as a component of flavorings and fragrances since it has an orange/lemon-like odor. Limonene has been shown to inhibit rat mammary and other tumor development http://www.ncbi.nlm.nih.gov/pubmed?term=Tsuda limonene 2004 Tsuda et al., 2004. Being an excellent solvent of cholesterol, d-limonene also has been used clinically to dissolve cholesterol-containing gallstones. Because of its gastric acid neutralizing effect and its support of normal peristalsis, it has also been used for relief of heartburn http://www.ncbi.nlm.nih.gov/pubmed/18072821 Sun, 2007.
Producing the flavoring substance limonene in our beer might result in a fresh, lemon-like taste on the one hand. On the other hand, we might have beneficial effects on health such as preventive activity against cancer, dissolution of gallstones and relief of heartburn.
Background and principles
Limonene is a cyclic terpene and a major constituent of several citrus oils (orange, lemon, mandarin, lime and grapefruit). It is a chiral liquid with the molecular mass of 136.24 g/mol. The (R)-enantiomer smells like oranges and is content of many fruits, while the (S)-enantionmer has a piney odor http://www.ncbi.nlm.nih.gov/pubmed/11605760 Fietzek, 2001. Therefore D-Limonene ((+)-Limonene, (R)-enantiomer) is used as a component of flavorings and fragrances.
Biosynthesis
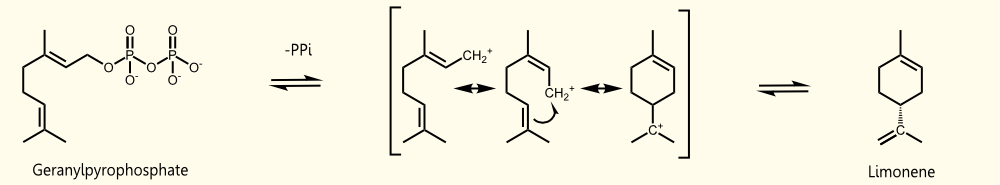
Limonene is produced by limonene synthase which uses geranyl pyrophosphate (GPP) as educt which is the universal precursor of monoterpenoids. (+)-limonene synthase from Citrus limon consists of 606 aminoacids (EC=4.2.3.20) and catalyzes the following reaction: Geranyl pyrophosphate = (+)-(4R)-limonene + diphosphate.
Saccharomyces cerevisiae produces geranyl pyrophosphate via the mevalonate pathway where it occurs exclusively as an intermediate of farnesyl pyrophosphate (FPP) synthesis http://www.ncbi.nlm.nih.gov/pubmed/17096665 Oswald et al., 2007. It has been established that S. cerevisiae has enough free GPP to be used by exogenous monoterpene synthases to produce monoterpenes under laboratory and vinification conditions [[http://www.ncbi.nlm.nih.gov/pubmed/18155949 Herrero et al., 2008], [http://www.ncbi.nlm.nih.gov/pubmed/17096665 Oswald et al., 2007]].
The molecular and physiological effects of limonene

Because of its pleasant Citrus flavour, D-Limonene is widely used as a flavor and fragrance additive. Therefore it is listed in the Code of Federal Regulations as generally recognized as safe (GRAS) http://www.fda.gov/downloads/Food/FoodIngredientsPackaging/GenerallyRecognizedasSafeGRAS/GRASListings/UCM264589.pdf FDA for a flavoring agent and can be found in common food items such as fruit juices, soft drinks, baked goods, ice cream, and pudding in typical concentrations of 50 ppm till 2,500 ppm, respectively http://www.ncbi.nlm.nih.gov/pubmed/18072821 Sun, 2007. Hence the daily consume of D-Limonene in a normal lifestyle is 0,27 mg/kg body weight http://www.mri.bund.de/fileadmin/Institute/PBE/Sekundaere_Pflanzenstoffe/Monoterpene.pdf. . and does not pose a mutagenic, carcinogenic, or nephrotoxic risk to humans.
As natural compound of Plants Limonene has practical advantages with regard to availability, suitability for oral apllication, regulatory approval and mechanisms of action.
It has been shown to inhibit rat mammary and other tumor development by Apoptosis induction and modulation of oncogene signal transduction http://www.ncbi.nlm.nih.gov/pubmed?term=Tsuda limonene 2004 Tsuda, 2004.
Furthermore It is used as excellent solvent of cholesterol, therefore d-limonene has been used clinically to dissolve cholesterol-containing gallstones. Because of its gastric acid neutralizing effect and its support of normal peristalsis, it also has been used for relief of heartburn http://www.ncbi.nlm.nih.gov/pubmed/18072821 Sun, 2007.
Results
BioBricks
[http://partsregistry.org/Part:BBa_K801060 BBa_K801060]
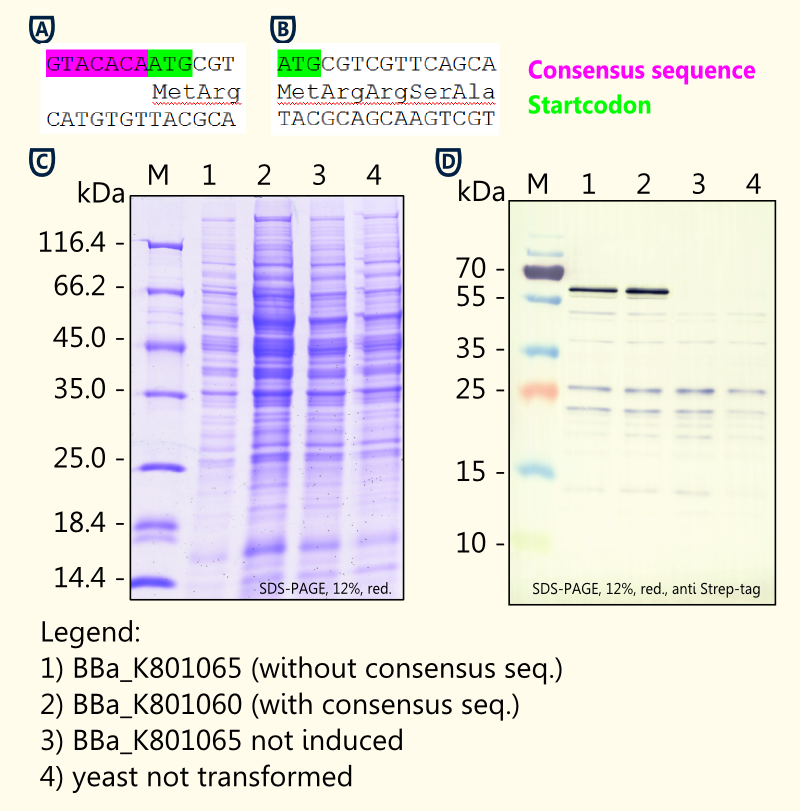
(+)-Limonene synthase 1 with Strep-tag and yeast consensus sequence.
This part contains the (+)-Limonene Synthase 1 of Citrus limon. It is preceeded by the yeast consensus sequence for improved expression and carries a C-Terminal Strep-Tag for purification or detection by westernblot. It is an improved version of BBa_I742111.
RFC 25 compatible
Further information:
- [http://www.ncbi.nlm.nih.gov/nucleotide/21435702 NCBI]
- UniProt entry: [http://www.uniprot.org/uniprot/Q8L5K3 Q8L5K3]
- E.C. Number: [http://enzyme.expasy.org/EC/4.2.3.20 4.2.3.20]
- Origin of the enzyme: Citrus limon
[http://partsregistry.org/Part:BBa_K801061 BBa_K801061]
(+)-Limonene synthase 1 coding region from Citrus limon.
Improved version of BBa_I742111.
Does not contain stop codon. Needs to be used with RFC 25.
Further information:
- [http://www.ncbi.nlm.nih.gov/nucleotide/21435702 NCBI]
- UniProt entry: [http://www.uniprot.org/uniprot/Q8L5K3 Q8L5K3]
- E.C. Number: [http://enzyme.expasy.org/EC/4.2.3.20 4.2.3.20]
- Origin of the enzyme: Citrus limon
[http://partsregistry.org/Part:BBa_K801062 BBa_K801062]
(+)-limonene synthase 1 expression cassette for yeast.
This part can be used to express Citrus limon (+)-limonene synthase 1 in yeast. The expression is controlled by TEF1 promoter and CYC1 terminator.
Further information:
- [http://www.ncbi.nlm.nih.gov/nucleotide/21435702 NCBI]
- UniProt entry: [http://www.uniprot.org/uniprot/Q8L5K3 Q8L5K3]
- E.C. Number: [http://enzyme.expasy.org/EC/4.2.3.20 4.2.3.20]
- Origin of the enzyme: Citrus limon
[http://partsregistry.org/Part:BBa_K801063 BBa_K801063]
(+)-limonene synthase 1 expression cassette for yeast.
This part can be used to express Citrus limon (+)-limonene synthase 1 in yeast. The expression is controlled by yeast TEF1 promoter and yeast TEF1 terminator.
Further information:
- [http://www.ncbi.nlm.nih.gov/nucleotide/21435702 NCBI]
- UniProt entry: [http://www.uniprot.org/uniprot/Q8L5K3 Q8L5K3]
- E.C. Number: [http://enzyme.expasy.org/EC/4.2.3.20 4.2.3.20]
- Origin of the enzyme: Citrus limon
[http://partsregistry.org/Part:BBa_K801064 BBa_K801064]
(+)-limonene synthase 1 expression cassette for yeast.
This part can be used to express Citrus limon (+)-limonene synthase 1 in yeast. The expression is controlled by yeast TEF2 promoter and yeast CYC1 terminator.
Further information:
- [http://www.ncbi.nlm.nih.gov/nucleotide/21435702 NCBI]
- UniProt entry: [http://www.uniprot.org/uniprot/Q8L5K3 Q8L5K3]
- E.C. Number: [http://enzyme.expasy.org/EC/4.2.3.20 4.2.3.20]
- Origin of the enzyme: Citrus limon
[http://partsregistry.org/Part:BBa_K801065 BBa_K801065]
(+)-Limonene synthase 1 with Strep-Tag
This part contains the coding region of (+)-limonene synthase from Citrus limon with a C-terminal Strep-tag. This part is based on BBa_K801061.
RFC 25 compatible
Further information:
- [http://www.ncbi.nlm.nih.gov/nucleotide/21435702 NCBI]
- UniProt entry: [http://www.uniprot.org/uniprot/Q8L5K3 Q8L5K3]
- E.C. Number: [http://enzyme.expasy.org/EC/4.2.3.20 4.2.3.20]
- Origin of the enzyme: Citrus limon
[http://partsregistry.org/Part:BBa_K801066 BBa_K801066]
(+)-Limonene synthase 1 with yeast consensus sequence for improved expression.
This part is second version of BBa_K801060. It contains (+)-limonene synthase and the consensus sequence for enhanced expression in yeast. It does not contain a C-terminal Strep tag as BBa_K801060. The sequence does not contain a stop codon so that RFC 25 has to be used.
RFC 25 compatible
Further information:
- [http://www.ncbi.nlm.nih.gov/nucleotide/21435702 NCBI]
- UniProt entry: [http://www.uniprot.org/uniprot/Q8L5K3 Q8L5K3]
- E.C. Number: [http://enzyme.expasy.org/EC/4.2.3.20 4.2.3.20]
- Origin of the enzyme: Citrus limon
Characterization
Gel Picture of finished construct
Investigation of the yeast consensus sequence
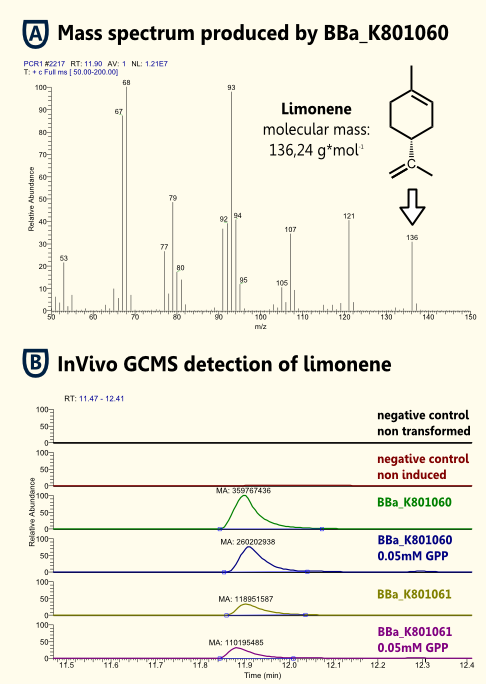
In vitro detection of limonene
To test functionality of purified Limonenesynthase in vitro we used an optimized protocol of an Enzyme Assay with previous Extraction of http://www.ncbi.nlm.nih.gov/pubmed/17662687 Landmann et al, 2007. The Enzyme Assay was carried out in Tris-HCl buffer with Glycerol, DTT and Cofactors. An identified amount of substrate (geranyl pyrophosphate) and purified recombinant limonene synthase. The mixture was extracted with pentane, dried with Sodiumsulfate and reduced under a stream of nitrogen. The pentane extracts were analyzed with gas chromatography-mass spectrometry ("5890 Series II GC" coupled to a "Finnigan Mat 55 S MS") to identify the enzymatically synthesized products.
In vivo detection of limonene
Because Limonene is volatile, [http://www.ncbi.nlm.nih.gov/pubmed/21310602 [Misawa, 2011]] we expected an arbitrarily amount of Limonene outside the cells in the supernatant. To verify this predication we could detect Limonene via Headspace (SPME needle) GC-MS in the yeast cell culture supernatant.
Detection of limonene in beer
A first atempt to use our genetically engineered yeasts to brew a first SynBio Beer were conducted using a transient transfection with a constitutive promoter. The drawback is that in the gyle the selection pressure is not preserved and the loss of the plasmid is possible.
Three liters of gyle were inoculated with 100ml of a stationary yeast culture grown in YPD that was transiently transfected with a plasmid harboring a constitutive expression cassette for the limonene synthase.
We analyzed this first beer for limonene content via Headspace (SPME needle) GC-MS. Unfortunately we could not yet proof a significant difference between our limonene containing beer and the negative control beer. This might be due to a lost of the plasmid which encodes limonene synthase. We will try to integrate the limonene synthase expression cassette into the genome of yeast in order to repeat the experiment.
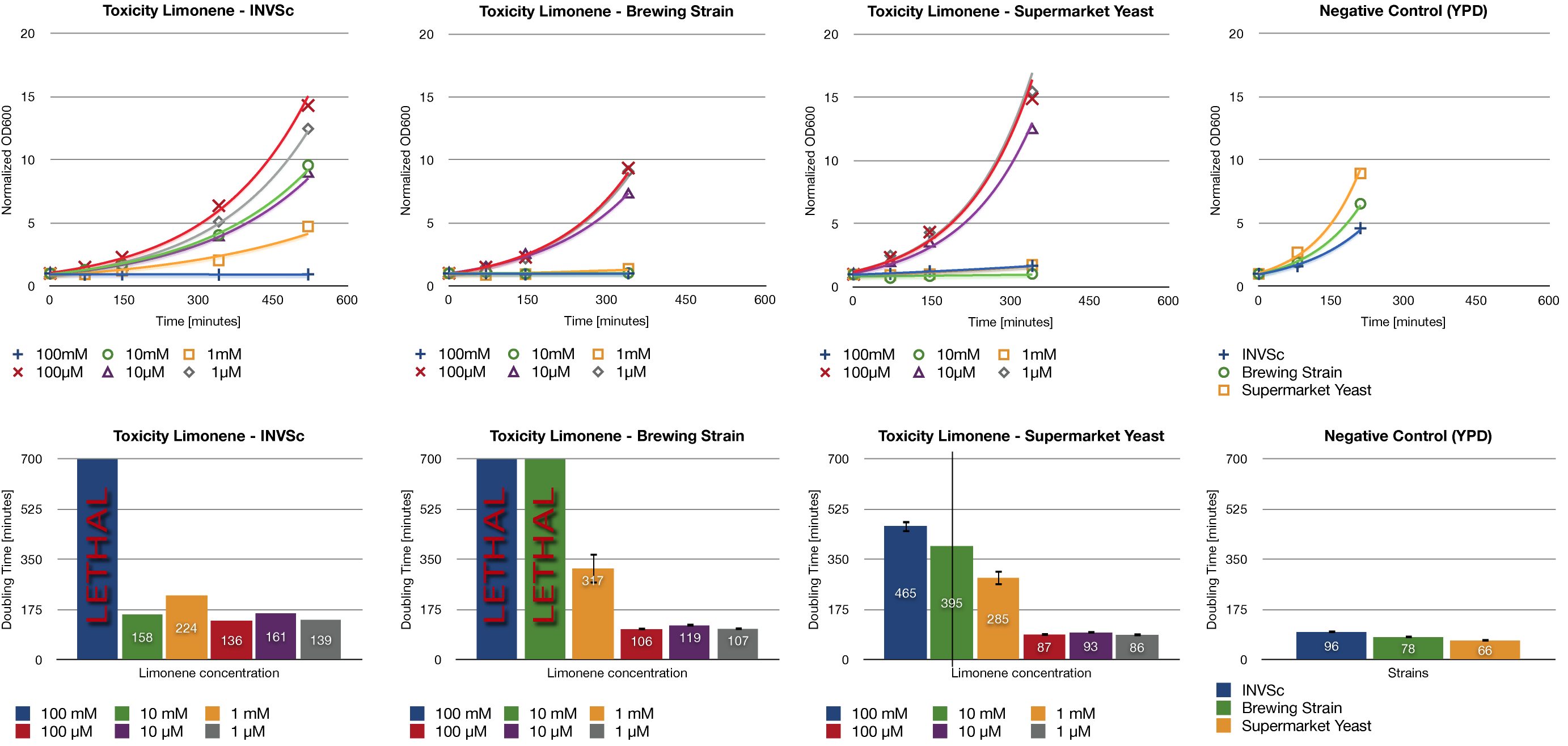
Toxicity Assay
To establish whether limonene has an effect on yeast cells , we inoculated three different yeast strains with different concentrations of limonene. Limonene was added to the medium and the used yeast strains were the laboratory strain INVSc1, a strain which is used for brewing beer and a strain which can be purchased in a supermarket.
Limonene at high concentrations affects the growth of yeast cells. We could show an inhibition of growth at 1 mM and even a lethal effect at 100 mM. At lower concentrations (1 µM, 10 µM, 100 µM) no inhibition could be observed. The growth rates of yeast cells which were incubated with low concentrations of limonene do not show a difference compared to the negative control (incubation with YPD without limonene).
The in vivo GCMS detection of limonene [B] displayed a concentration of 50 µM. Hence the amount of limonene we will produce with the modified yeast will not reach a toxic concentration at all.
References
- http://www.ncbi.nlm.nih.gov/pubmed/11605760 Fietzek et al., 2001 Fietzek, C., Hermle, T., Rosenstiel, W., Schurig, V. (2001) Chiral discrimination of limonene by use of beta-cyclodextrin-coated quartz-crystal-microbalances (QCMs) and data evaluation by artificial neuronal networks. Fresenius J Anal Chem., 371(1):58-63.
- http://www.ncbi.nlm.nih.gov/pubmed/18155949 Herrero et al., 2008 Herrero, O., Ram ́on, D., and Orejas, M. (2008). Engineering the Saccharomyces cerevisiae isoprenoid pathway for de novo production of aromatic monoterpenes in wine. Metab Eng, 10(2):78–86.
- http://www.ncbi.nlm.nih.gov/pubmed/17662687 Landmann et al., 2007 Landmann, C., Fink, B., Festner, M., Dregus, M., Engel, K.-H., and Schwab, W. (2007). Cloning and functional characterization of three terpene synthases from lavender (Lavandula angustifolia). Arch Biochem Biophys, 465(2):417–29.
- http://www.ncbi.nlm.nih.gov/pubmed/12084056 Lücker et al., 2002 Lücker, J., El Tamer, M. K., Schwab, W., Verstappen, F. W. A., van der Plas, L. H. W., Bouwmeester, H. J., and Verhoeven, H. A. (2002). Monoterpene biosynthesis in lemon (Citrus limon). cDNA isolation and functional analysis of four monoterpene synthases. Eur J Biochem, 269(13):3160–71.
- http://www.ncbi.nlm.nih.gov/pubmed/17096665 Oswald et al., 2007 Oswald, M., Fischer, M., Dirninger, N., and Karst, F. (2007). Monoterpenoid biosynthesis in Saccharomyces cerevisiae. FEMS Yeast Res, 7(3):413–21.
- http://www.ncbi.nlm.nih.gov/pubmed/20675444 Rico et al., 2010 Rico, J., Pardo, E., and Orejas, M. (2010). Enhanced production of a plant monoterpene by overexpression of the 3-hydroxy-3-methylglutaryl coenzyme a reductase catalytic domain in Saccharomyces cerevisiae. Appl Environ Microbiol, 76(19):6449–54.
- http://www.ncbi.nlm.nih.gov/pubmed/18072821 Sun, 2007 Sun, J. (2007). D-limonene: safety and clinical applications. Altern Med Rev, 12(3):259–64.
- http://www.ncbi.nlm.nih.gov/pubmed?term=Tsuda limonene 2004 Tsuda et al., 2004 Tsuda, H., Ohshima, Y., Nomoto, H., Fujita, K., Matsuda, E., Iigo, M., Takasuka, N., Moore, MA. (2004). Cancer prevention by natural compounds. Drug Metab Pharmacokinet. 19(4):245-63.
- http://www.mri.bund.de/fileadmin/Institute/PBE/Sekundaere_Pflanzenstoffe/Monoterpene.pdf Watzl, 2002 Watzl, B. (2002). Monoterpene. Ernährungs-Umschau 49 Heft 8. 322-324.
- http://www.ncbi.nlm.nih.gov/pubmed/9724535 Williams et al., 1998 Williams, D. C., McGarvey, D. J., Katahira, E. J., and Croteau, R. (1998). Truncation of limonene synthase preprotein provides a fully active ’pseudomature’ form of this monoterpene cyclase and reveals the function of the amino-terminal arginine pair. Biochemistry, 37(35):12213–20.
- http://www.fda.gov/downloads/Food/FoodIngredientsPackaging/GenerallyRecognizedasSafeGRAS/GRASListings/UCM264589.pdf FDA
 "
"