Contents |
Title
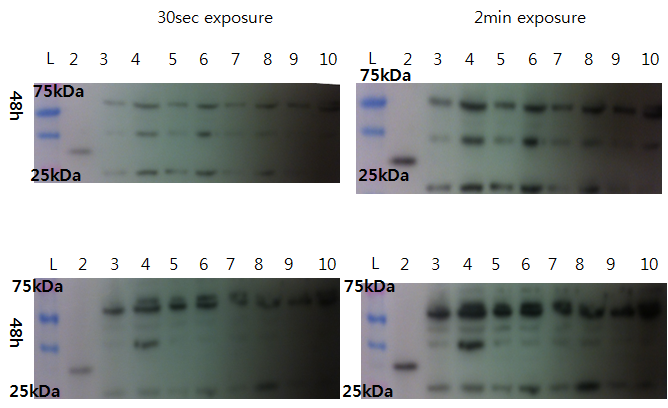
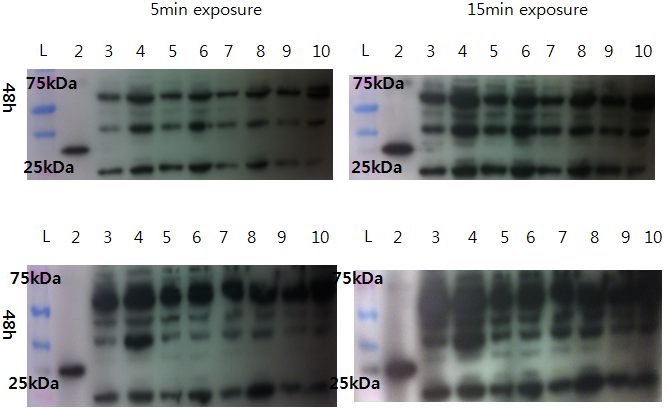
Western Blot check for Lovtap-VP16 expression
With comparison of 48h transfected CHO-cell and non-transfected CHO cells, we try to see the difference. Result: Couldn't find difference.
- Loading plan
- Lane 1: Ladder
- Lane 2: VP16 only
- Lane 3: 10 microL Non-T CHO
- Lane 4: 20 microL Sample 1(100% lovtap)
- Lane 5: 10 microL Non-T CHO
- Lane 6: 20 Lovtap Sample 2(95% lovtap + 5% GFP)
- Lane 7: 10 microL Non-T CHO
- Lane 8: 20 Lovtap Sample 3(100% lovtap)
- Lane 9: 10 microL Non-T CHO
- Lane 10: 20 microL Sample 3(100% lovtap)
FACS at EPFL facility (GRAPHS! please!) Transfection of LovTAP for microscopy at BIOPS. 6 tubes.
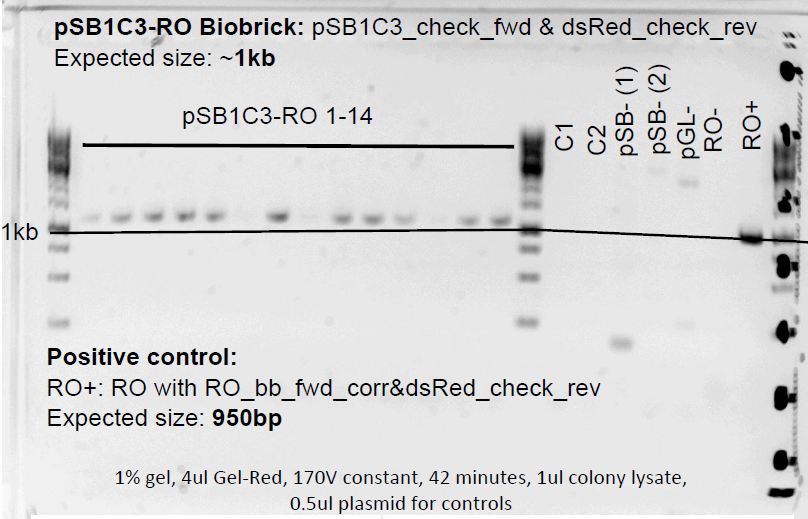
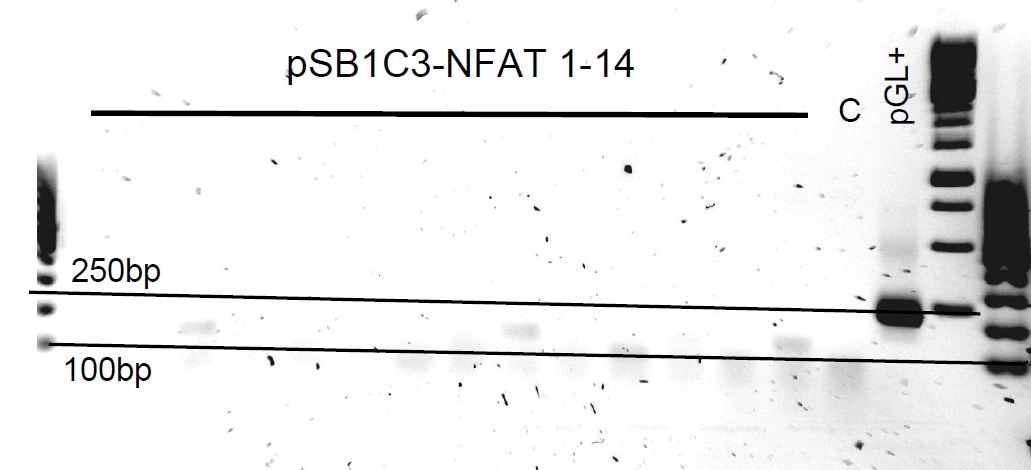
pSB1C3-NFAT and pSB1C3-RO colony PCR
Protocol: Colony PCR
Preparation: Prepare a falcon tube for each colony you wish to test,
- Add 3ul of LB in each tube
- Add appropriate amount of the appropriate antibiotic
- Add 5 to 10ul of Lyse & GO (lysis reagent) into PCR tubes (one for each colony)
- Pick a colony on the plate
- Dip it in the falcon tube, don't drop the tip!
- Dip the same tip in the PCR tube with Lyse&GO.
- Repeat for each colony
- Put the PCR tubes in the PCR machine and select the Lyse&GO programm (10min at 95°C)
- Incubate the falcon tubes at 37°C.
PCR:
Chose an appropriate primer set to test your colonies, for example a forward primer specific to the backbone you want and a reverse primer specific to your gene of interest.
Volumes for a 20ul reaction, add in this order. (Multiply by the number of reaction you want to do +1)
| Reagent | Volume [μl] |
|---|---|
| Water | 20-x |
| HF-Buffer (5x) | 4 |
| DMSO (optional) | 0.6 |
| dNTPs | 0.4 |
| Forward primer (50μM) | 0.2 |
| Reverse primer (50μM) | 0.2 |
| Phusion HF polymerase | 0.2 |
Add 19ul of master mix to each tube and then add 1ul of colony lysate in the right tube (keep track of which colony is numbered how and in which tube!).
Controls controls controls!! Don't forget to have a positive control (for example, amplify your gene of interest from its original plasmid, this will directly give you the approximate size of the bands you expect), negative controls for your primer set and if colonies grew on you control plates test them with the same primer set as your test colonies.
Protocol: PCR
PCR is a reaction that makes it possible (and relatively easy) to amplify
a certain region of DNA. The first step is the selection of that region
(and the design of the relevant primers). Primer design can be done by hand, or by
using our Primer Design Helper. Once
done, order the primers (in our case, we ordered from them [http://www.idtdna.com/ IDT]).
When you've received the primers, prepare them and make sure you've got your PCR kit (we used the "Phusion® High-Fidelity DNA Polymerase"). Start preparing your master mix, the composition for one tube is:
1X Mastermix 20μl reaction, add in this order
| Reagent | Volume [μl] |
|---|---|
| Water | Complete to total volume of 20μl |
| HF-Buffer (5x) | 4 |
| DMSO (optional) | 0.6 |
| dNTPs | 0.4 |
| Forward primer (50μM) | 0.2 |
| Reverse primer (50μM) | 0.2 |
| Template (10ng/μl) | 0.5 |
| Phusion HF polymerase | 0.2 |
Prepare one or two extra tubes-worth of reagent (you'll use some liquid on the walls of your tips).
Once you've finished, you should run the resulting products on a gel to check if everything went as planned.
Tips
- Thaw the HF-Buffer, DMSO and dNTPs before making the mastermix.
- Avoid taking the Phusion-HF polymerase out of the freezer (only take it out briefly when you need to add it).
- If the reactions have different primers and/or template, add the polymerase right after the dNTPs, split the mastermix and add the rest.
- Don't forget positive and negative controls
- Primers should have similar Tms (less than 5°C).
- Primer Tm calculation is a less exact science than it should be (just test several tools and compare their results). If you're not sure what the correct Tm is, consider using a gradient PCR.
- Avoid primers with strong secondary structures.
- PCR can introduce mutations. Don't forget to sequence your final product (this could be your final plasmid): you really don't want to lose a few weeks because of a "corrupt" plasmid.
Colonies grew on both ligation plates. So 14 colonies were picked in each pSB1C3-RO and pSB1C3-NFAT plate and also on the control plates for control.
Negative controls:
RO:
- RO-: RO with pSB1C3_check_fwd & dsRed_check_rev
- pSB-(2): pSB1C3 with pSB1C3_check_fwd & dsRed_check_rev
NFAT:
- pGL-: pGL4.30 with pSB1C3_check_fwd & NFAT_bb_rev_v3
- pSB-(1): pSB1C3 with pSB1C3_check_fwd & NFAT_bb_rev_v3
- pGL+: pGL4.30 with NFAT_bb_fwd & NFAT_bb_rev_v3 (positive control)
pSB1C3-NFAT failed. We also observed red culture tubes in the evening which confirms that the colonies that grew contain the original pSB1C3-RFP plasmid and not the ligation we want.
 "
"