Team:Peking/Modeling/Luminesensor/Orthogonality
From 2012.igem.org
| Line 50: | Line 50: | ||
<p></p> | <p></p> | ||
<ul class="refer"><li id="ref1"> | <ul class="refer"><li id="ref1"> | ||
| - | 1. Zoltowski, B.D., Crane, B.R.(2008)Light Activation of the LOV Protein Vivid Generates a Rapidly Exchanging Dimer.<i>Biochemistry</i>, 47: 7012: 7019 | + | 1. Zoltowski, B.D., Crane, B.R.(2008). Light Activation of the LOV Protein Vivid Generates a Rapidly Exchanging Dimer.<i>Biochemistry</i>, 47: 7012: 7019 |
</li><li id="ref2"> | </li><li id="ref2"> | ||
2. Mohana-Borges, R., Pacheco, A.B., Sousa, F.J., Foguel, D., Almeida, D.F., and Silva, J.L. (2000). LexA repressor forms stable dimers in solution. The role of specific dna in tightening protein-protein interactions. <i>J. Biol. Chem.</i>, 275: 4708: 4712 | 2. Mohana-Borges, R., Pacheco, A.B., Sousa, F.J., Foguel, D., Almeida, D.F., and Silva, J.L. (2000). LexA repressor forms stable dimers in solution. The role of specific dna in tightening protein-protein interactions. <i>J. Biol. Chem.</i>, 275: 4708: 4712 | ||
Revision as of 13:07, 26 September 2012
Orthogonal Test in silico
To modularize the genetic system, our Luminesensor is expected to be bio-orthogonal with the origin system in bacteria. LexA, a natural element from the lactin-SOS system in bacteria may cause unexpected crosstalk. In order to remove this obstacle on the application prospects of our Luminesensor, we use LexA408 instead of the wild-type LexA. LexA408 and LexA are bio-orthogonal with each other since the sequence of the binding sites have variations (See Characterization).
By adding several nodes into the network, we constructed modeling for orthogonality test:
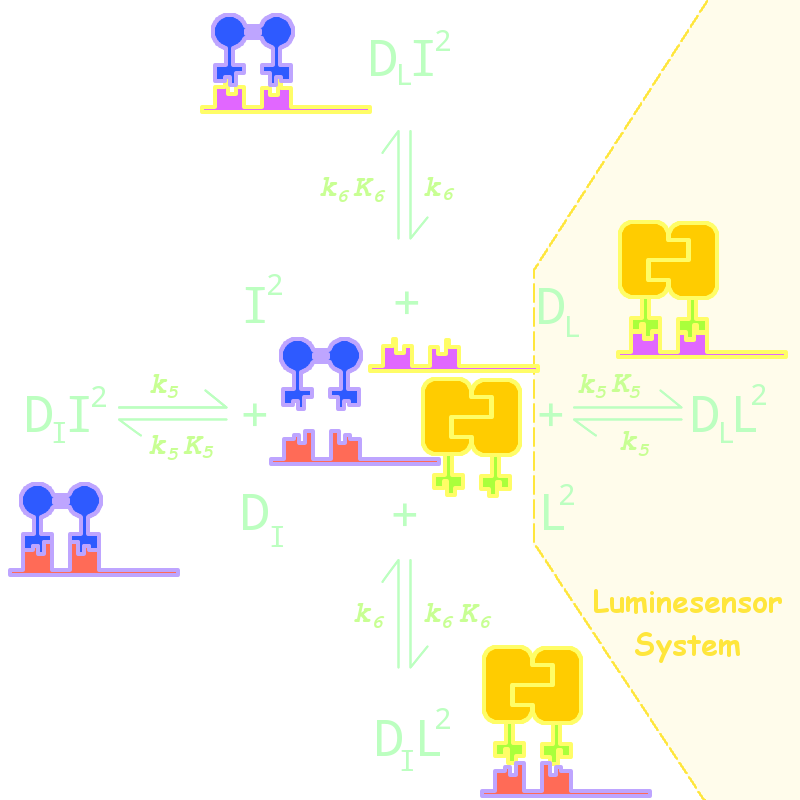
Fig 5. Kinetic Network for Orthogonal Analysis
where
- L denotes Luminesensor
- I denotes the inner wild LexA
- DL denotes the specific DNA binding site to Luminesensor
- DI denotes the specific DNA binding site to wild LexA
The parameters are estimated as following:
| Parameter | Value | Unit | Description |
| k6 | 1.x10-4 | s-1 | dimered LexA releasing rate constant from non-specific binding site |
| K6 | 1.x10-2 | (n mol/L)-1 | dimered non-specific binding equilibrium constant |
Tab 2. Reaction Parameters for Orthogonal Test
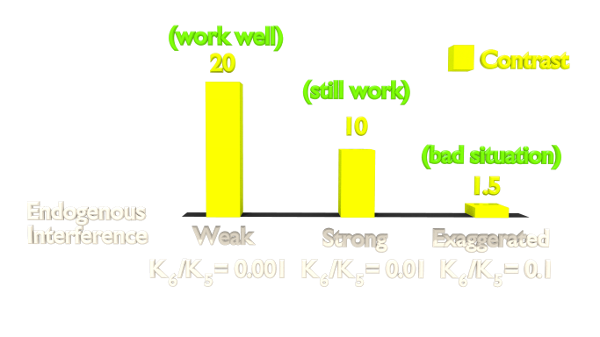
Fig 6. Orthogonal Test Result.
The result shows that the contrast is highly related to the orthogonality. As our Luminesensor is orthogonal to the endogerous LexA system, our system still works well in bacteria with endogenous LexA (See Characterization).
Reference
- 1. Zoltowski, B.D., Crane, B.R.(2008). Light Activation of the LOV Protein Vivid Generates a Rapidly Exchanging Dimer.Biochemistry, 47: 7012: 7019
- 2. Mohana-Borges, R., Pacheco, A.B., Sousa, F.J., Foguel, D., Almeida, D.F., and Silva, J.L. (2000). LexA repressor forms stable dimers in solution. The role of specific dna in tightening protein-protein interactions. J. Biol. Chem., 275: 4708: 4712
- 3. Zoltowski, B.D., Vaccaro, B., and Crane, B.R. (2009). Mechanism-based tuning of a LOV domain photoreceptor. Nat. Chem. Biol. 5: 827: 834
- 4. Dmitrova, M., Younes-Cauet, G., Oertel-Buchheit, P., Porte, D., Schnarr, M., Granger-Schnarr, M.(1998) A new LexA-based genetic system for monitoring and analyzing protein heterodimerization in Escherichia coli. Mol. Gen. Genet., 257: 205: 212
 "
"