Team:TU Munich/Project/Caffeine
From 2012.igem.org
(→References) |
(→Toxicity Assay) |
||
| Line 164: | Line 164: | ||
==== Toxicity Assay ==== | ==== Toxicity Assay ==== | ||
<div> | <div> | ||
| + | |||
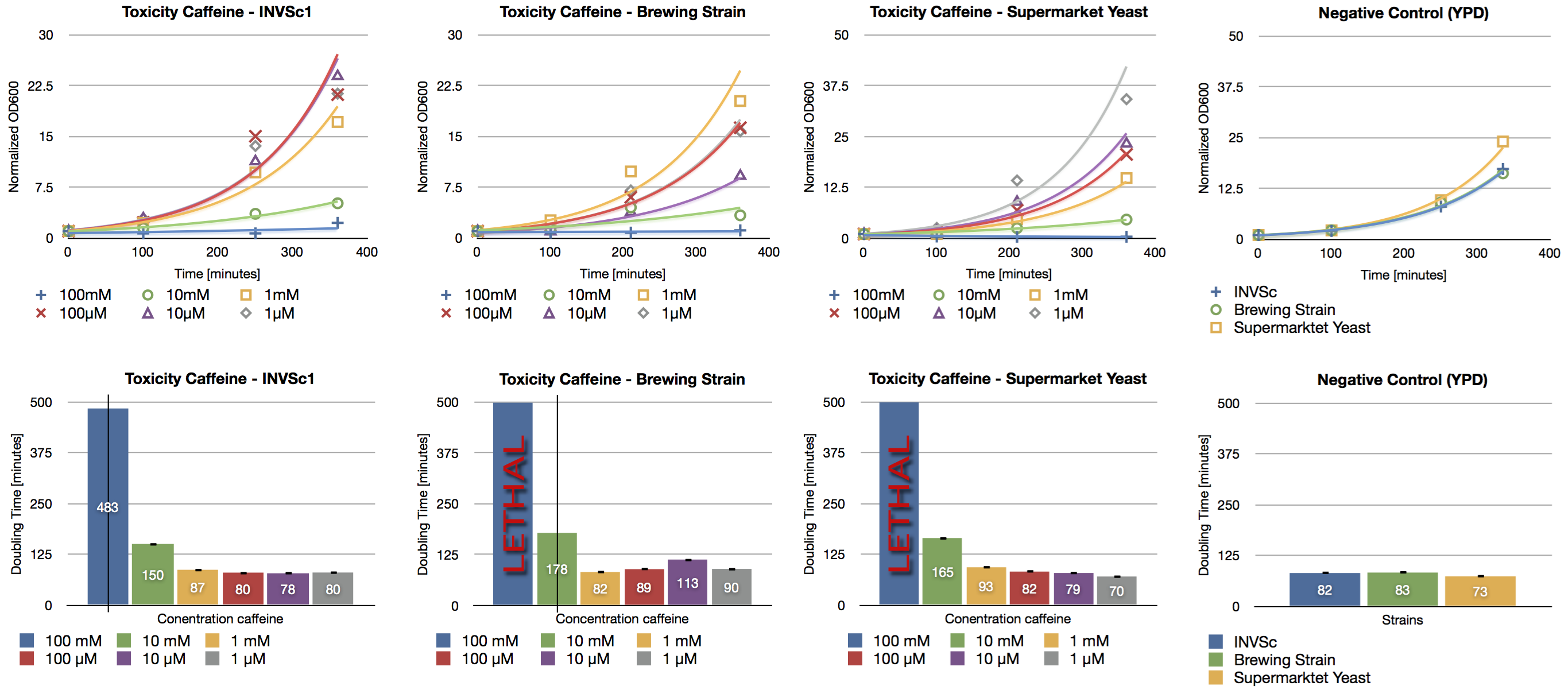
| + | Since high doses of caffeine (> 10 mM) have mutagenic effects on yeast (Kuranda et al., 2006) we investigated the effect of different caffeine concentrations on different yeast strains. | ||
| + | The used yeast strains were the laboratory strain INVSc1, a strain which is used for brewing beer and a strain which can be purchased in a supermarket. Caffeine was added to the YPD medium in concentrations from 1 µM up to 100 mM and the growth rate was measured after a defined period of time. | ||
| + | |||
| + | We confirmed the toxic effect of caffeine on yeast at the concentration of 100 mM and the growth inhibition at the concentration of 10 mM. Furthermore we showed the correlation between decreasing caffeine concentration and growth rate. Cells incubated with caffeine concentrations in the range of micro molar showed a similar growth rate than the negative control (incubation without caffeine). | ||
| + | |||
| + | |||
[[File:TUM12_Toxicitiy_Caffeine.png|600px|thumb|center|Evaluation of the Toxicity Assay for Caffeine.]] | [[File:TUM12_Toxicitiy_Caffeine.png|600px|thumb|center|Evaluation of the Toxicity Assay for Caffeine.]] | ||
Revision as of 22:38, 25 September 2012



Caffeine
In contrast, caffeine wards of drowsiness and already enjoys great popularity as additive in a multitude of beverages. As hops is essential to the brewing process, omitting is not an option. This makes caffeine a desirable agent to counteract the soporific effect of beer.
We were successful in expressing (SDS- Page and Western Blot Analysis) all three genes which are necessary for caffeine biosynthesis (in plants) in our yeast stem INVSc1 after having cloned the genes in the new yeast expression vector pTUM100. In order to proove the functionality of the enzymes, we performed an enzyme assay and tried to detect the resulting caffeine and other intermediates by the use of LCMS. The three single genes were submitted as biobricks x, y and z.
Furthermore, we generated the caffeine synthesis biobrick, which containes all three neccessary genes at once - each with its own promoter and terminator, whereas the strength of the promoters was individually chosen.
In order to investigate the growth of our yeast cells under the influence of caffeine at different concentrations, we also performed a toxicity assay.
It has already been achieved to produce caffeine in tobacco plants (Uefuji et al., 2005; Yun- Soo Kim et al., 2007) and in in an in vitro assay, but has never been performed in yeast.
Background and principles
Biosynthesis
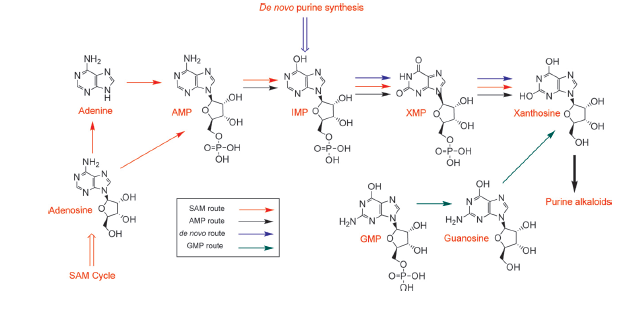
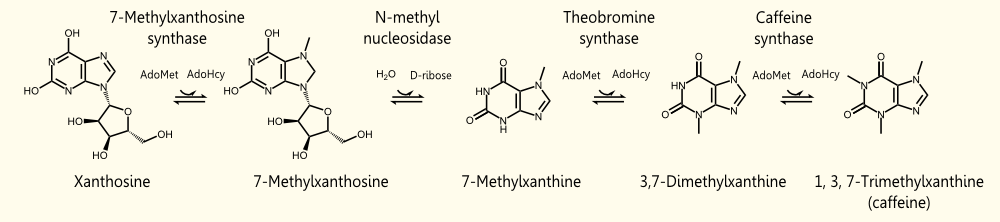
The biosynthetic pathway of caffeine (1,3,7 Trimethylxanthine) starts with xanthosine, which is a natural component of the purine- metabolism of all organisms. Necessary for its production are three distinct N- methyl transferases and one nucleosidase, whereupon it has not been totally elucidated whether the nucleosidase reaction is catalyzed by any purine nucleosidase or by the first N- methyl transferase of the reaction cascade shown in the picture (but the latter assumption is favoured (H. Ashihara et al., 2008), because an in vitro synthesis of caffeine with the three N- methyl transferases has already been shown). The enzyme caffeine synthase (last reaction step) can catalyze both, the conversion of 7- methyl xanthine to theobromine and the methylation of theobromine to caffeine. This is true, indeed, but Uefuji et al. (2003) showed, that the affinity to 7- methyl xanthosine is less than one sixth of that of CaMXMT1 (there are two isoformes of CaMXMT), so it is much better to express both enzymes. One can also see the Km values for the required enzymes in this paper - it shows that the substrate affinity decreases continiously towards the endpoint (caffeine), "making the reaction proceed irreversibly and stepwise" (Uefuji et al., 2003, p.377).
The chemical compound xanthosine is produced via at least four different routes, shown in the picture "xanthosine routes". To improve caffeine production, these pathways could be a possible target for metabolic engineering in the future.
During the degradation of caffeine, it is demethylated to theophylline by 7N-demethylase (main pathway). The decreased rate of this reaction is the reason for accumulating caffeine in the plant. Afterwards, theophylline is degraded to xanthine via 3-methylxanthine and xanthine enters the conventional purine catabolism pathway (degradation to CO2 and NH3) (see H. Ashihara et al., 2008, p. 846). This catabolistic pathway is another possible target for metabolic engineering to increase the amount of caffeine.
The molecular and physiological effects of caffeine
Caffeine is a purine-alkaloid and its biosynthesis is known in coffee plants and tea plants, for example. Its chemical structure is similar to the ribonucleoside adenosine. Hence it can block specific receptors in the hypothalamus. Adenosine binding leads to decreased neurotransmitter-release and therefore decreased neuron activity. Biological background is to beware the brain of overexertion by inducing sleep and that is the reason for using caffeine to stay awake, since it is antagonizing adenosine and increases the neuron activity by reducing the effects of adenosine. On average, one cup (150ml) of coffee contains about 50 - 130 mg caffeine and one cup of tea 25 - 90 mg.At higher doses (1g), caffeine leads to higher pulse rates and hyperactivity, but until that, the alcohol will already have done its work...
Moreover, caffeine was shown to decrease the growth of E. Coli and Yeast reversibly as of a concentration of 0.1% by acting as a mutagen (Putrament et al., On the Specificity of Caffeine Effects, MGG, 1972), but previous caffeine synthesis experiments (see below) have only led to a concentration of about 5 µg/g (per g fresh weight of tobacco leaves), so we do not expect to reach critical concentrations and the amounts of caffeine in coffee or tea (leading to physiological effects) is usually a little bit lower.
Results
BioBricks
All the generated BioBricks are basing upon the mRNA sequences having been isolated out of coffea arabica by Hiroshi Sano et al., 2003, and registered at [http://www.ncbi.nlm.nih.gov/pubmed/ NCBI] (see numbers below). However, these sequences were modificated in several ways, to make them iGEM compatible and improve the usage in general, respectively.
Modifications:
- the 5' UTR and 3' UTR of the original sequences were removed
- the yeast consensus sequence for improved ribosome binding (TACACA) was added 5' of the start codon ATG
- according to N- end rule and the yeast consensus sequence for improved ribosome binding, the first triplet after ATG (GAG) was exchanged with TCT (serine), to optimize both, protein stability and mRNA translation. This decision was made after proofing the 3D- structure of the enzyme CaDXMT1. Due to the fact, that the the first two residues of the amino acid sequence are not shown in the crystalized structure (probably because of high flexibility), we chose to exchange this amino acid, for it is probably not that necessary for the uptake of the ligands (uniprot entry further shows, that it is not immediately involved in ligand binding in one of the three enzymes). Because of the high similarity of the enzyme- sequences, we also exchanged this amino acid in the enzymes CaXMT1 and CaMXMT1.
- we added a c- terminal strep-tag for purification and detection
- the remaining coding sequence was extended with the standard RFC10 prefix and suffix, respectively
- at last we made an optimization of the coding sequences with respect to the codon usage and mRNA structures (online tool of gene- synthesis company)
- remove of all critical restriction sites (RFC10 and RFC25)
All mentionend methyltransferases use SAM als methyl- donor and are located in the cytoplasm of the plants. Furthermore they exist as homodimers, being also able to form heterodimers with each other (see [http://www.brenda-enzymes.info Brenda], also for further characteristics). The temperature and pH optimum of all three enzymes is quite similar between 20°C - 37°C and 7,5 - 8,5, respectively (beer brewing: slightly acid).
In vivo, this irreversibility is realized by continuously increasing Km values (Km(CaXMT1) < Km(CaMXMT1) < Km(CaDXMT1)); as soon as a certain amount of theobromine is available, the caffeine synthesis can go on.
[http://partsregistry.org/Part:BBa_K801070 BBa_K801070]
NCBI- Access number of original gene sequence: AB048793
This RFC10 compatible BioBrick encodes the enzyme CaXMT1 (xanthosine N-methyltransferase 1 of coffea arabica). It is catalyzing the first reaction step of the caffeine biosynthesis pathway by methylation of the initial substrate xanthosine, resulting in 7- methylxanthine. The enzyme thereby uses SAM (S- adenosyl methionine) as cosubstrate (methyl- donor).
Further information:
- UniProt entry: Q9AVK0
- E.C. Number: 2.1.1.158
- PDB: 3D- Structure: [http://www.pdb.org/pdb/explore/explore.do?structureId=2eg5 C. canephora CaXMT1]
- Theoretical molecular weight (without posttranslational modifications): 42998,4 Da (ProtParam)
Probable posttranslational modifications:
- acetylation at serine (second amino acid)
- O-GlcNAc modifikation at several positions
- phosphorylation at several positions
(see [http://2d.bjmu.edu.cn/show2d/Proteomics%20tools.asp ProteomicsTools])
[http://partsregistry.org/Part:BBa_K801071 BBa_K801071]
NCBI- Access number of original gene sequence: AB048794
This RFC10 compatible biobrick encodes the enzyme CaMXMT1 (7-methylxanthine N-methyltransferase of coffea arabica). It is catalyzing the third reaction step of the caffeine biosynthesis pathway by methylation of the substrate 7- methylxanthine, resulting in 3,7- dimethylxanthine (=theobromine). The enzyme thereby uses SAM (S- adenosyl methionine) as cosubstrate (methyl- donor).
Further information:
- UniProt entry: Q9AVJ9
- E.C. Number: 2.1.1.159
- Theoretical molecular weight (without posttranslational modifications): 43903,3 Da (ProtParam)
Probable posttranslational modifications:
- acetylation at serine (second amino acid)
- O-GlcNAc modifikation at several positions
- phosphorylation at several positions
(see [http://2d.bjmu.edu.cn/show2d/Proteomics%20tools.asp ProteomicsTools])
[http://partsregistry.org/Part:BBa_K801072 BBa_K801072]
NCBI- Access number of original gene sequence: AB084125
This RFC10 compatible BioBrick encodes the enzyme CaDXMT1 (3,7-dimethylxanthine N-methyltransferase of coffea arabica). It catalyzes the third reaction step of the caffeine biosynthesis pathway by methylation of the substrate 3,7- dimethylxanthine, resulting in 1,3,7- trimethylxanthine (=caffeine). The enzyme thereby uses SAM (S- adenosyl methionine) as cosubstrate (methyl- donor).
Further information:
- UniProt entry: Q8H0D2
- 2.1.1.160
- PDB: 3D- Structure: [http://www.pdb.org/pdb/explore/explore.do?structureId=2efj C. canephora CaDXMT1]
- Theoretical molecular weight (without posttranslational modifications): 44473,7 Da (ProtParam)
Probable posttranslational modifications:
- acetylation at serine (second amino acid)
- O-GlcNAc modifikation at several positions
- phosphorylation at several positions
(see [http://2d.bjmu.edu.cn/show2d/Proteomics%20tools.asp ProteomicsTools])
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K801073 BBa_K801073]
This BioBrick is the generator for the enzyme xanthosine N-methyltransferase 1 (CaXMT1). It is regulated by the constitutive promoter Tef2, which is a strong yeast promoter. The used terminator is Adh1, a widely used yeast terminator. The Tef2 promoter was preferred to the Tef1 promoter (which is even stronger) in order to limit metabolic stress, which could result in a positive selection of natural mutants (after genome integration).
This BioBrick is also a part of the "caffeine synthesis device" (see below).
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K801074 BBa_K801074]
This BioBrick is the generator for the enzyme 7-methylxanthine N-methyltransferase 1(CaMXMT1) (= theobromine synthase). It is regulated by the constitutive promoter Tef1, which is one of the strongest yeast promoters. The used terminator is Adh1, as it is among the other expression cassettes. The choice of the strong Tef1 promoter was made, because the in vivo biosynthesis of caffeine is an irreversible reaction, which is ensured by continously increasing Km and vmax values of the single enzymes. As soon as a certain amount of theobromine is available, the caffeine synthesis can go on. To support this irreversible, stepwise caffeine synthisis, we used the strongest promoter for this enzyme, to establish high concentrations of the caffeine precursor theobromine.
This BioBrick is also a part of the "caffeine synthesis device" (see below).
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K801075 BBa_K801075]
This BioBrick is the generator for the enzyme 3,7-dimethylxanthine N-methyltransferase 1 (CaDXMT1) (= caffeine synthase), i.e. the last enzyme envolved in caffeine biosynthesis. It is regulated by the constitutive promoter Tef2, which is also used at the expression cassette of CaXMT1. The used terminator is Adh1, as it is among the other expression cassettes. The choice of the strong Tef1 promoter was made, because the in vivo biosynthesis of caffeine is an irreversible reaction, which is ensured by continously increasing Km and vmax values of the single enzymes. As soon as a certain amount of theobromine is available, the caffeine synthesis can go on. The choice for this promoter was made to prevent metabolic stress reactions and because of the required irreversibility of the caffeine production (see above).
This BioBrick is also a part of the "caffeine synthesis device" (see below).
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K801076 BBa_K801076]
This BioBrick is a generator for the first two enzymes envolved in caffeine biosynthesis: methylxanthosine N-methyltransferase 1 and 7- methylxanthine N-methyltransferase 1. It is made up of the two single generators (see above).
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K801077 BBa_K801077]
Characterization
Gene expression
The expression of the three neccessary enzymes involved in caffeine biosynthesis will be prooved by SDS page and Western Blot analysis, making use of the Strep-tag II and a detection system based on two antibodies: StrepMAB-Classic (from mouse) and an Anti-Mouse-alk. phosphatase conjugate.
Enzyme-function
First of all, the function of the three enzymes will be demonstrated in vitro in a combinated reaction batch, containing all three enzymes. After adding the initial substrate xanthosine, we will detect the synthesized caffeine after organic extraction and HPLC separation (densitometrically).
Function of expression cartridge and membran-permeability of compounds
At first, the fuction of our expression cartridge will be prooved by Western Blot analysis, as mentioned above. To investigate wether both, the initial substrate xanthosine and the final product caffeine are able to penetrate the yeast cell wall, we will apply the substrate xanthosine to a cell suspension, in which the cells contain our created "caffeine synthesis biobrick" with the constitutive promoters Tef1 and Tef2. Caffeine presence will be detected as mentioned above.
Toxicity Assay
Since high doses of caffeine (> 10 mM) have mutagenic effects on yeast (Kuranda et al., 2006) we investigated the effect of different caffeine concentrations on different yeast strains. The used yeast strains were the laboratory strain INVSc1, a strain which is used for brewing beer and a strain which can be purchased in a supermarket. Caffeine was added to the YPD medium in concentrations from 1 µM up to 100 mM and the growth rate was measured after a defined period of time.
We confirmed the toxic effect of caffeine on yeast at the concentration of 100 mM and the growth inhibition at the concentration of 10 mM. Furthermore we showed the correlation between decreasing caffeine concentration and growth rate. Cells incubated with caffeine concentrations in the range of micro molar showed a similar growth rate than the negative control (incubation without caffeine).
References
- Kuranda, K., Leberre, V., Sokol, S., Palamarczyk, G., François, J., 2006. Investigating the caffeine effects in the yeast Saccharomyces cerevisiae brings new insights into the connection between TOR, PKC and Ras/cAMP signalling pathways. Molecular Microbiology 61, 1147–1166.
- Putrament et al., On the Specificity of Caffeine Effects, MGG, 1972
- H. Uefuji et al., Plant Physiology, 2003, Vol. 132, pp. 372–380
- H. Uefuji et al., Plant Molecular Biology, 2005, Vol. 59, p. 221–227
- H. Ashihara et al., Phytochemistry, 2008, Vol. 69, p. 841–856
- Yun-Soo Kim, Hiroshi Sano, Phytochemistry, 2008, Vol. 69, p. 882–888
- Franco et al., 2012
- Fisone G, Borgkvist A, Usiello A (2004). "Caffeine as a psychomotor stimulant: mechanism of action". Cell. Mol. Life Sci. 61 (7–8): 857–72.
older blocks
- please integrate the informations into the other blocks and delete the sequences here (copy them into the registry)
General remarks and issues
Analytical Methods
- Detection and quantification of the produced caffeine can be performed by the use of HPLC. Uefuji et al., 2005; describe those and other relevant methods in this context.
- Proof of the gene- expression (of the necessary methyl- transferases) could be accomplished by standard methods (Western Blot, see also Uefuji et al., 2005; and Yun- Soo Kim and Hiroshi Sano, 2007;)
 "
"