Team:CU-Boulder/Project
From 2012.igem.org
Mjacobs8891 (Talk | contribs) |
|||
| Line 5: | Line 5: | ||
https://static.igem.org/mediawiki/2012/a/a4/Fig9.png | https://static.igem.org/mediawiki/2012/a/a4/Fig9.png | ||
| - | + | Lux CDE - synthesizes fatty acid with acyl CoA as starting substrate | |
| + | <br> | ||
| + | <br> | ||
| + | LuxAB- dimer that oxides aldehyde and FMNH2 with reducing O2 and produces light! Light comes from oxidation of FMNH2, which is a chromophore | ||
| + | <br> | ||
| + | <br> | ||
| + | Lux G - Not an essential gene for the system but increases the light produced by reducing FMN+ | ||
| + | |||
== Project Details== | == Project Details== | ||
Revision as of 17:18, 25 June 2012

Contents |
Overall project

Lux CDE - synthesizes fatty acid with acyl CoA as starting substrate
LuxAB- dimer that oxides aldehyde and FMNH2 with reducing O2 and produces light! Light comes from oxidation of FMNH2, which is a chromophore
Lux G - Not an essential gene for the system but increases the light produced by reducing FMN+
Project Details
Project 2
Quorum Sensing in LuxR containing Bacteria
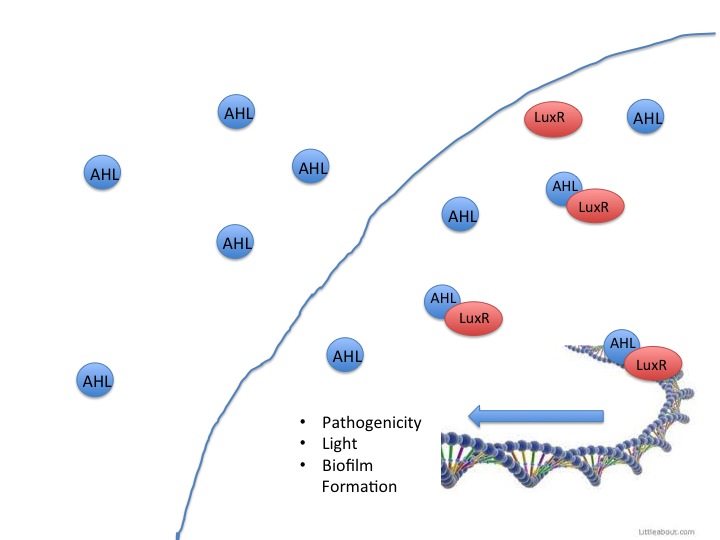
This year the CU iGEM team has harnessed the quorum sensing factors endogenous in the Salmonella enterica serovar typhimurium LT2 strain to detect AHLs produced by other bacteria. Neither Salmonella nor E. coli have been shown to produce detectable levels of AHLs, so they were model organisms for the detection of other AHL producing bacteria such as Vibrio fisheri, or pathogenic bacteria such as Yersinia pestis. AHLs are small molecules synthesized by most gram negative bacteria, and are able to freely diffuse throughout the membrane. When the concentration of AHL producing bacteria in a specific area increases, the total concentration of AHLs diffusing into the cytoplasm also increases. Once a threshold level of AHLs are in the cytosol, transcription factors like the Vibrio fisheri LuxR or Salmonella enterica SdiA activate gene synthesis for quorum factors to be produced. In pathogenic bacteria, these signals have been shown to activate transcription of pathogenic factors.
Detection and silencing of AHL producing bacteria
The SdiA transcription factor has been shown to be more sensitive to lower concentrations and a greater diversity of AHLs than its LuxR homolog. We took advantage of this extra-sensitivity to create a detection system for AHL producing bacteria, while additionally attaching the detection system to the secretion of potent AHLases such as the Aiia enzyme, and a reporter protein RFP. Using this construct we tested whether we could disrupt the quorum sensing signal AHLs in order to keep them from reaching the threshold concentration to activate the less sensitive LuxR receptor. The V. fisheri species and other pathogenic bacteria use the LuxR receptor, therefore inhibition of AHLs would keep V. fisheri from producing light. Keeping the V. fisheri from producing light is an analogous model to keeping pathogenic bacteria from being stimulated by AHLs to release their pathogenesis factors.
Inhibiting quorum sensing in bacteria has been shown to not only inhibit the transcription of pathogenesis factors, but also to keep biofilms from forming. In the history of iGEM teams have used secreted enzymes to digest biofilms that have already been made, but our use of quorum sensing inhibitors have gone a step further, and are being tested in their ability to keep bacteria from making biofilms in the first place.
 "
"