Team:Cambridge/Safety
From 2012.igem.org
(→iGEM safety questions) |
Pdmallaband (Talk | contribs) |
||
| Line 10: | Line 10: | ||
===iGEM safety questions=== | ===iGEM safety questions=== | ||
| - | '''1. Would any of your project ideas raise safety issues in terms of:''' | + | <span style="color:green">'''1. Would any of your project ideas raise safety issues in terms of:'''</span> |
| - | *researcher safety, | + | <span style="color:blue">*researcher safety,</span> |
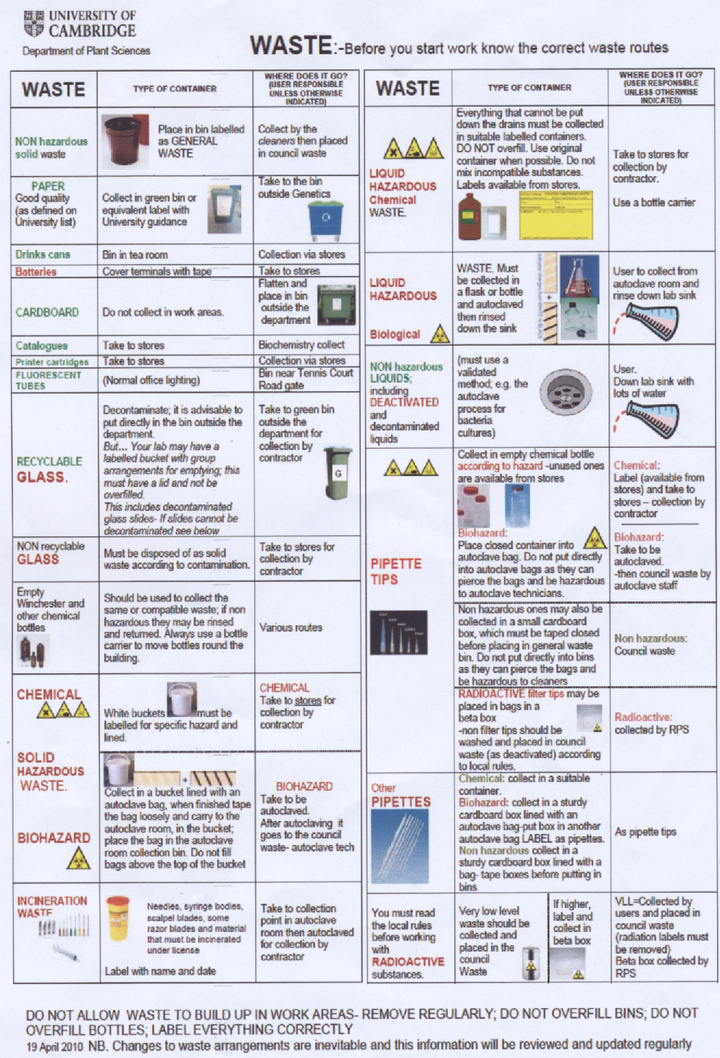
Our laboratory is only authorised to use Biosafety level 1 (non-pathogenic) bacteria and we therefore consider our project to be '''harmless to researchers''' as none of our experiments have any known action in increasing the pathogenicity of the bacteria we use or the host range. It is departmental policy however to treat all bacteria as potential pathogens and to take suitable safety precautions. We understand that it is essential to wear suitable PPE (personal protective equipment) at all times, in our experiments this usually means labcoats and gloves. All laboratory equipment and bacterial cultures are to be decontaminated by autoclaving. A copy of the departmental regulations for waste disposal is available at the bottom of this page. | Our laboratory is only authorised to use Biosafety level 1 (non-pathogenic) bacteria and we therefore consider our project to be '''harmless to researchers''' as none of our experiments have any known action in increasing the pathogenicity of the bacteria we use or the host range. It is departmental policy however to treat all bacteria as potential pathogens and to take suitable safety precautions. We understand that it is essential to wear suitable PPE (personal protective equipment) at all times, in our experiments this usually means labcoats and gloves. All laboratory equipment and bacterial cultures are to be decontaminated by autoclaving. A copy of the departmental regulations for waste disposal is available at the bottom of this page. | ||
| Line 21: | Line 21: | ||
We anticipate that elements of our project could be used for a wide variety of purposes and these may include the use of potentially harmful substances. Any future teams would need to consider the safety implications of their project on a case by case basis, though our project should not raise any safety concerns. | We anticipate that elements of our project could be used for a wide variety of purposes and these may include the use of potentially harmful substances. Any future teams would need to consider the safety implications of their project on a case by case basis, though our project should not raise any safety concerns. | ||
| - | *public safety, | + | <span style="color:blue">*public safety,</span> |
We understand public apprehension surrounding the use of genetically modified bacteria, in the unlikely event that members of the public came into contact with the bacteria used in our project we anticipate that there would be '''no threat to public safety''' as we are using non-pathogenic strains. | We understand public apprehension surrounding the use of genetically modified bacteria, in the unlikely event that members of the public came into contact with the bacteria used in our project we anticipate that there would be '''no threat to public safety''' as we are using non-pathogenic strains. | ||
| - | *environmental safety? | + | <span style="color:blue">*environmental safety? </span> |
While we appreciate that pathogenic strains of the species we are using exist, we are using disabled, non-pathogenic strains that should otherwise interact in an identical manner with the environment. We therefore anticipate that any released bacteria would be '''disadvantaged''' and are not expected to survive outside of the favorable conditions engineered in the lab. | While we appreciate that pathogenic strains of the species we are using exist, we are using disabled, non-pathogenic strains that should otherwise interact in an identical manner with the environment. We therefore anticipate that any released bacteria would be '''disadvantaged''' and are not expected to survive outside of the favorable conditions engineered in the lab. | ||
| Line 41: | Line 41: | ||
Please note that we are not currently implementing these strategies. While it is important to consider how we would prevent the spread of GMOs if we were to use them in the field, the measures discussed above will prevent their spread from the research environment. | Please note that we are not currently implementing these strategies. While it is important to consider how we would prevent the spread of GMOs if we were to use them in the field, the measures discussed above will prevent their spread from the research environment. | ||
| - | '''2. Do any of the new BioBrick parts (or devices) that you made this year raise any safety issues? If yes,''' | + | <span style="color:green">'''2. Do any of the new BioBrick parts (or devices) that you made this year raise any safety issues? If yes,'''</span> |
| - | *did you document these issues in the Registery? | + | <span style="color:blue">*did you document these issues in the Registery?</span> |
| - | *how did you manage to handle the safety issue? | + | <span style="color:blue">*how did you manage to handle the safety issue?</span> |
| - | *how could other teams learn from your experience | + | <span style="color:blue">*how could other teams learn from your experience</span> |
Our biobricks are designed to create a biological system whereby a bacterial cell takes in some substance to be measured and this induces a light output. None of our biobricks increase either the pathogenicity of the bacteria used or the range of usable hosts. | Our biobricks are designed to create a biological system whereby a bacterial cell takes in some substance to be measured and this induces a light output. None of our biobricks increase either the pathogenicity of the bacteria used or the range of usable hosts. | ||
| Line 52: | Line 52: | ||
Nevertheless, we are remaining vigilant in ensuring that no GMOs come into contact with either researchers or the wider world. | Nevertheless, we are remaining vigilant in ensuring that no GMOs come into contact with either researchers or the wider world. | ||
| - | '''3. Is there a local biosafety group, committee or review board at your institution?''' | + | <span style="color:green">'''3. Is there a local biosafety group, committee or review board at your institution?'''</span> |
| - | *If yes, what does your local biosafety group think about your project? | + | <span style="color:blue">*If yes, what does your local biosafety group think about your project?</span> |
Departmental Codes of Practice for GM organisms were also consulted before we began our project. We have thus been cleared to work with bacteria that have been classified as being "unlikely to cause human disease". | Departmental Codes of Practice for GM organisms were also consulted before we began our project. We have thus been cleared to work with bacteria that have been classified as being "unlikely to cause human disease". | ||
| Line 61: | Line 61: | ||
As we are a UK team there are also national biosafety regulations ([http://www.hse.gov.uk/biosafety/gmo/law.htm here]) that we were made aware of before beginning our project. We have complied with all of these guidelines, and hence have worked well within the law. | As we are a UK team there are also national biosafety regulations ([http://www.hse.gov.uk/biosafety/gmo/law.htm here]) that we were made aware of before beginning our project. We have complied with all of these guidelines, and hence have worked well within the law. | ||
| - | *If no, which specific biosafety rules or guidelines do you have to consider in your country | + | <span style="color:blue">*If no, which specific biosafety rules or guidelines do you have to consider in your country</span> |
| - | '''4. Do you have any other ideas how to deal with safety issues that could be useful for future iGEM competitions? How could parts, devices and systems be made even safer through biosafety engineering? | + | N/A |
| + | |||
| + | |||
| + | <span style="color:green">'''4. Do you have any other ideas how to deal with safety issues that could be useful for future iGEM competitions? How could parts, devices and systems be made even safer through biosafety engineering?</span> | ||
Biosafety was a major consideration when planning our project and many (we feel) quite brilliant project ideas were discarded because they posed significant safety risks or the procedures it would be necessary to implement to work safely were unfeasible within the time scale of the project. Project ideas that would have required the widespread or uncontrolled release of our bacteria into the environment were also discarded. | Biosafety was a major consideration when planning our project and many (we feel) quite brilliant project ideas were discarded because they posed significant safety risks or the procedures it would be necessary to implement to work safely were unfeasible within the time scale of the project. Project ideas that would have required the widespread or uncontrolled release of our bacteria into the environment were also discarded. | ||
Revision as of 18:00, 24 September 2012
Safety
N.B. This page is a work in process and will be added to and improved over the course of the project.
iGEM safety questions
1. Would any of your project ideas raise safety issues in terms of: *researcher safety,
Our laboratory is only authorised to use Biosafety level 1 (non-pathogenic) bacteria and we therefore consider our project to be harmless to researchers as none of our experiments have any known action in increasing the pathogenicity of the bacteria we use or the host range. It is departmental policy however to treat all bacteria as potential pathogens and to take suitable safety precautions. We understand that it is essential to wear suitable PPE (personal protective equipment) at all times, in our experiments this usually means labcoats and gloves. All laboratory equipment and bacterial cultures are to be decontaminated by autoclaving. A copy of the departmental regulations for waste disposal is available at the bottom of this page.
Several techniques used in the course of this project call for the use of potentially hazardous substances which range from causing minor skin irritations to being toxic if ingested. For this reason we have created risk assessment pages for each of the protocols used in the course of our research. These are not intended to be used instead of departmental safety procedures, but outline the major concerns for each protocol for reference by future teams. MSDS sheets for reagents used in the course of our project are also available on this wiki.
Sodium Fluoride is hazardous and was necessary for testing our fluoride riboswitch. Full departmental risk assessments were completed before working with this substance and a brief risk assessment outlining the main dangers can be found on this wiki (here) as well as a MSDS.
We anticipate that elements of our project could be used for a wide variety of purposes and these may include the use of potentially harmful substances. Any future teams would need to consider the safety implications of their project on a case by case basis, though our project should not raise any safety concerns.
*public safety,
We understand public apprehension surrounding the use of genetically modified bacteria, in the unlikely event that members of the public came into contact with the bacteria used in our project we anticipate that there would be no threat to public safety as we are using non-pathogenic strains.
*environmental safety?
While we appreciate that pathogenic strains of the species we are using exist, we are using disabled, non-pathogenic strains that should otherwise interact in an identical manner with the environment. We therefore anticipate that any released bacteria would be disadvantaged and are not expected to survive outside of the favorable conditions engineered in the lab.
Should the goals of our project come to fruition, there are additional safety factors that must be considered upon the implementation of our kit in the field. Our project must be able to remain within the bounds of current laws to be considered a success. Biocontainment of the bacteria used as the biosensors is therefore a high priority. Current systems are typically somewhat cumbersome and unreliable in their implementation - for example, sample tubes may have to be collected and then sterilized with boiling water. In order to ensure that no GMOs are released into the natural environment upon use of our system, we have devised several strategies that could be used to prevent release of viable bacteria from our kit:
- The storage cuvettes could contain a small container of bleach. Breaking a seal when the cuvette is finished with results in its release and causes destruction of the active bacteria. The cuvette can then be disposed of in general waste, with all GMOs inside destroyed.
- During sporulation, a unique sigma factor is activated. This acts upon promotors that bring about the sporulation response. Our cells could therefore be engineered to activate an internal clock upon sporulation that would remain activated after germination and cause the destruction of the cells after a certain number of generations.
- The cells could be engineered to require a complex substance that is only practically available in their medium. Should the cells stray from their medium, an autolysis gene would be activated and the cells would self destruct. This is analogous to the survival factors required by mammalian cells to prevent apoptosis.
Use of several (or, for preference, all) of these techniques should prevent unintentional spread of our GMOs. Taking a lesson from the development of antibiotic resistance in bacterial strains, we hope that with several failsafes such as the ones that we are proposing in place, the likelihood of any single cell surviving the entire series of biocontainment measures will fall to essentially zero.
Please note that we are not currently implementing these strategies. While it is important to consider how we would prevent the spread of GMOs if we were to use them in the field, the measures discussed above will prevent their spread from the research environment.
2. Do any of the new BioBrick parts (or devices) that you made this year raise any safety issues? If yes, *did you document these issues in the Registery? *how did you manage to handle the safety issue? *how could other teams learn from your experience
Our biobricks are designed to create a biological system whereby a bacterial cell takes in some substance to be measured and this induces a light output. None of our biobricks increase either the pathogenicity of the bacteria used or the range of usable hosts.
Our biobricks do not raise any safety issues.
Nevertheless, we are remaining vigilant in ensuring that no GMOs come into contact with either researchers or the wider world.
3. Is there a local biosafety group, committee or review board at your institution? *If yes, what does your local biosafety group think about your project?
Departmental Codes of Practice for GM organisms were also consulted before we began our project. We have thus been cleared to work with bacteria that have been classified as being "unlikely to cause human disease".
Our project and protocols have been reviewed and accepted by our advisers and the departmental safety officer as suitable.
As we are a UK team there are also national biosafety regulations ([http://www.hse.gov.uk/biosafety/gmo/law.htm here]) that we were made aware of before beginning our project. We have complied with all of these guidelines, and hence have worked well within the law.
*If no, which specific biosafety rules or guidelines do you have to consider in your country
N/A
4. Do you have any other ideas how to deal with safety issues that could be useful for future iGEM competitions? How could parts, devices and systems be made even safer through biosafety engineering?
Biosafety was a major consideration when planning our project and many (we feel) quite brilliant project ideas were discarded because they posed significant safety risks or the procedures it would be necessary to implement to work safely were unfeasible within the time scale of the project. Project ideas that would have required the widespread or uncontrolled release of our bacteria into the environment were also discarded.
We recommend that future teams consider following this planning procedure, as the real world applications proposed by many past teams appear to fail to take into consideration the risks posed by the uncontrolled release of their GMOs into the wider environment.
We also recommend that future teams try to consider failsafes in the biocontainment aspects of their projects to reduce the risks of GMOs evolving resistance to biocontainment efforts.
Additional safety information
- Risk Assessments Risk assessments for any experiments we do and safety information for carrying out any of the protocols listed on this wiki.
- MSDS Sheets Materials Safety Data Sheets for the reagents used in the course of our project.
- Protocols Protocols used in the course of our project.
- Waste procedure summary A copy of several waste safety posters around the department:
 "
"