Team:Peking/Modeling/Channel
From 2012.igem.org
Nathan 1991 (Talk | contribs) m |
|||
| Line 12: | Line 12: | ||
<br/><br/>Functional the existing biobrick may be, there are two major concerns about it: </p> | <br/><br/>Functional the existing biobrick may be, there are two major concerns about it: </p> | ||
<ul> | <ul> | ||
| - | <li>1. Blue light absorption at 455nm (Fig. 1, Dark) | + | <li>1. Blue light absorption at 455nm (Fig. 1, Dark) suffers from a disability of penetrating tissues for further applications such as phototherapy, because of its short wavelength. |
<div class="floatC"> | <div class="floatC"> | ||
| - | <img src="" alt="figure1 | + | <img src="/wiki/images/0/09/Peking2012_Sensor_Future_Redshift.jpg" alt="figure1" style="width:500px" /> |
| - | <p class="description">Figure 1 | + | <p class="description" style="text-align:center;font-style:normal;">Figure 1. Absorption Spectrum of VVD protein</p> |
</div> | </div> | ||
</li> | </li> | ||
| Line 22: | Line 22: | ||
</ul> | </ul> | ||
<p> | <p> | ||
| - | Thus, the Peking iGEM 2012 team is greatly motivated by the thought of developing a red-shifted VVD domain for | + | Thus, the Peking iGEM 2012 team is greatly motivated by the thought of developing a red-shifted VVD domain for <i>Luminesensor</i>. And such a dual channel system will sure see a bright future in its complexity and diversity. |
</p> | </p> | ||
</div> | </div> | ||
| Line 32: | Line 32: | ||
</p> | </p> | ||
<div class="floatC"> | <div class="floatC"> | ||
| - | <img src="" alt="figure2 goes here" /> | + | <img src="/wiki/images/1/14/Peking2012_luminesensor_future_2.gif" alt="figure2 goes here" /> |
<p class="description">Figure 2 Vancancy in the pocket of VVD</p> | <p class="description">Figure 2 Vancancy in the pocket of VVD</p> | ||
</div> | </div> | ||
<div class="floatC"> | <div class="floatC"> | ||
| - | <img src="" alt="figure3 goes here" /> | + | <img src="/wiki/images/7/75/Peking2012_luminesensor_future_3.png" alt="figure3 goes here" style="width:400px"/> |
<p class="description">Figure 3 Molecular structure of FAD</p> | <p class="description">Figure 3 Molecular structure of FAD</p> | ||
</div> | </div> | ||
| Line 43: | Line 43: | ||
<h3 id="title3">Design</h3> | <h3 id="title3">Design</h3> | ||
<p> | <p> | ||
| - | The choice of the FAD analogue should have the following features: a red-shifted absorption spectrum and a substitution at 8 position. After selecting from of a pool of over 500 chemicals listed by similarity search on SciFinder, we identified 8- | + | The choice of the FAD analogue should have the following features: a red-shifted absorption spectrum and a substitution at 8 position. After selecting from of a pool of over 500 chemicals listed by similarity search on SciFinder, we identified 8-tetrahydropyrroriboflavin (Fig. 4) as a promising candidate for incorporation, which has a red shift of absorption at 510nm. |
</p> | </p> | ||
<div class="floatC"> | <div class="floatC"> | ||
| - | <img src="" alt="figure4 goes here" /> | + | <img src="/wiki/images/5/5c/Peking2012_luminesensor_future_4.png" alt="figure4 goes here" style="width:400px"/> |
| - | <p class="description">Figure 4 | + | <p class="description">Figure 4 Molecular Structure of 8-tetrahydropyrrole riboflavin</p> |
</div> | </div> | ||
<p> | <p> | ||
On the other hand, in order to attain bio-orthoganality and to efficiently accommodate the FAD analogue, necessary mutations should be done with the pocket of VVD. Molecular docking results (Fig. 5) indicate that this analogue interacts with the protein at four specific sites(residues marked in blue). These side chains serve as spatial hindrances in that they are within a distance of 3 angstrom (dash lines). And mutations of these residues will hopefully create enough space for the 8-substituted FAD analogue, in this case, a tetrahydropyrrole is at the 8 position. | On the other hand, in order to attain bio-orthoganality and to efficiently accommodate the FAD analogue, necessary mutations should be done with the pocket of VVD. Molecular docking results (Fig. 5) indicate that this analogue interacts with the protein at four specific sites(residues marked in blue). These side chains serve as spatial hindrances in that they are within a distance of 3 angstrom (dash lines). And mutations of these residues will hopefully create enough space for the 8-substituted FAD analogue, in this case, a tetrahydropyrrole is at the 8 position. | ||
| - | <div class="floatC"> | + | </p> |
| - | <img src="" alt="figure5 goes here" /> | + | <div class="floatC"> |
| - | <p class="description">Figure 5 | + | <img src="/wiki/images/c/c9/Peking2012_luminesensor_future_005.gif" alt="figure5 goes here" /> |
| + | <p class="description">Figure 5 Spatial interactions between FAD analogue and the pocket of VVD</p> | ||
</div> | </div> | ||
</div> | </div> | ||
Revision as of 07:33, 24 September 2012
Motivation
Light-oxygen-voltage (LOV) domain containing fusion proteins are validated photosensing devices that have been widely utilized and well characterized in the field of optogenetics. The use of Vivid (VVD) in the construction of an ultra-sensitive luminesensor (LexA-VVD) by Peking iGEM 2012 has opened the gate to a new generation of optogentics.
Functional the existing biobrick may be, there are two major concerns about it:
- 1. Blue light absorption at 455nm (Fig. 1, Dark) suffers from a disability of penetrating tissues for further applications such as phototherapy, because of its short wavelength.
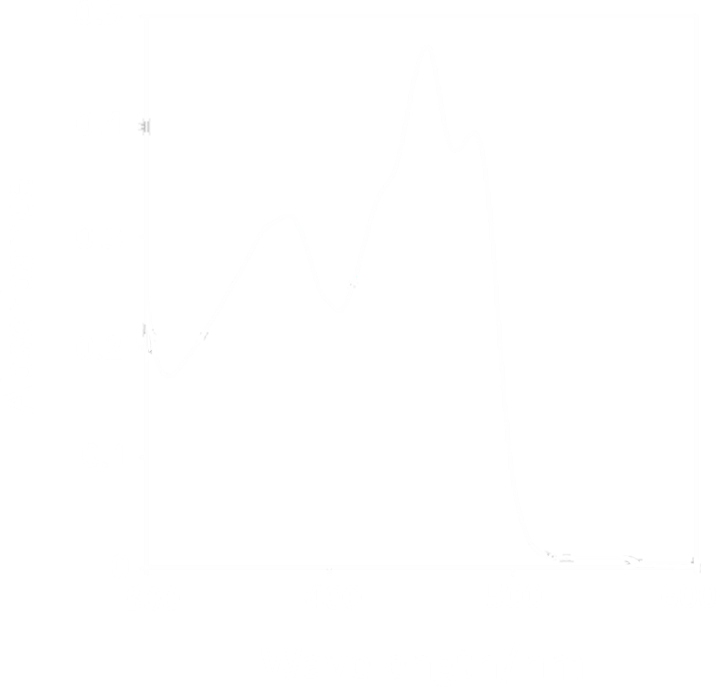
Figure 1. Absorption Spectrum of VVD protein
- 2. Mono-channel manipulation of cellular behavior is not enough for future extensions of more complexity
Thus, the Peking iGEM 2012 team is greatly motivated by the thought of developing a red-shifted VVD domain for Luminesensor. And such a dual channel system will sure see a bright future in its complexity and diversity.
Rationale
The spectroscopic characters of VVD is mostly determined by its chromophore—Flavin Adanine Dinucleotide (FAD) substrate. And thus any changes to the absorption spectrum should be focused on modifications to the conjugating substructure of FAD.
One essential prerequisite for the incorporation of an FAD analogue is that the VVD pocket is big enough to accommodate a structurally extended FAD. And the protein structure of VVD shows it’s possible for substitution in 8 position of riboflavin-- the light absorbing group of FAD (Fig. 2&3). We can see a large space, measured by dash lines (unit: Angstrom), is vacant for future incorporation of an 8-substituted FAD analogue.
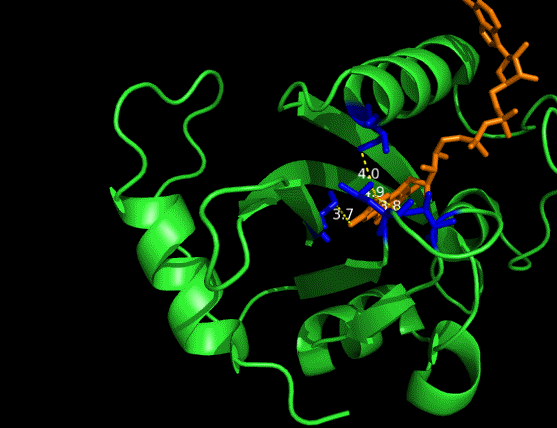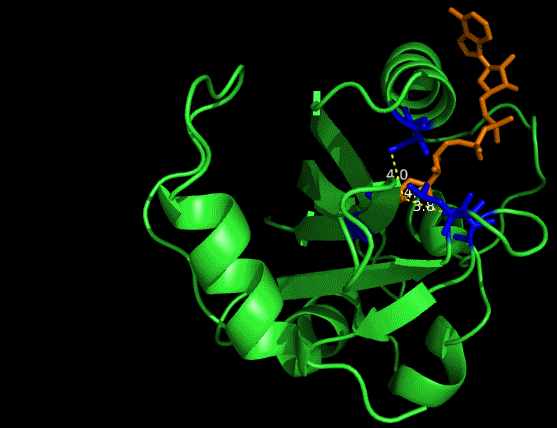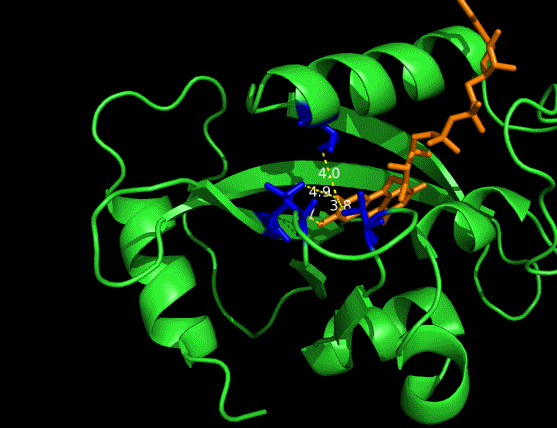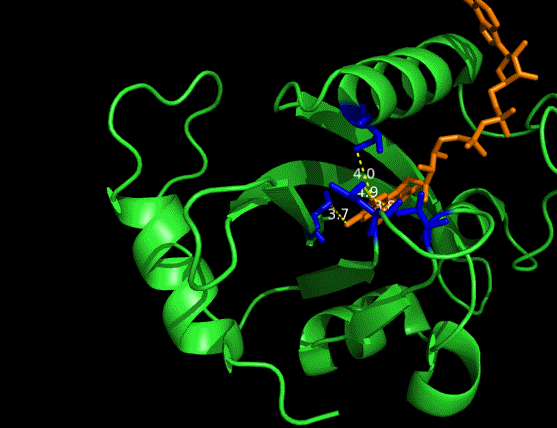
Figure 2 Vancancy in the pocket of VVD
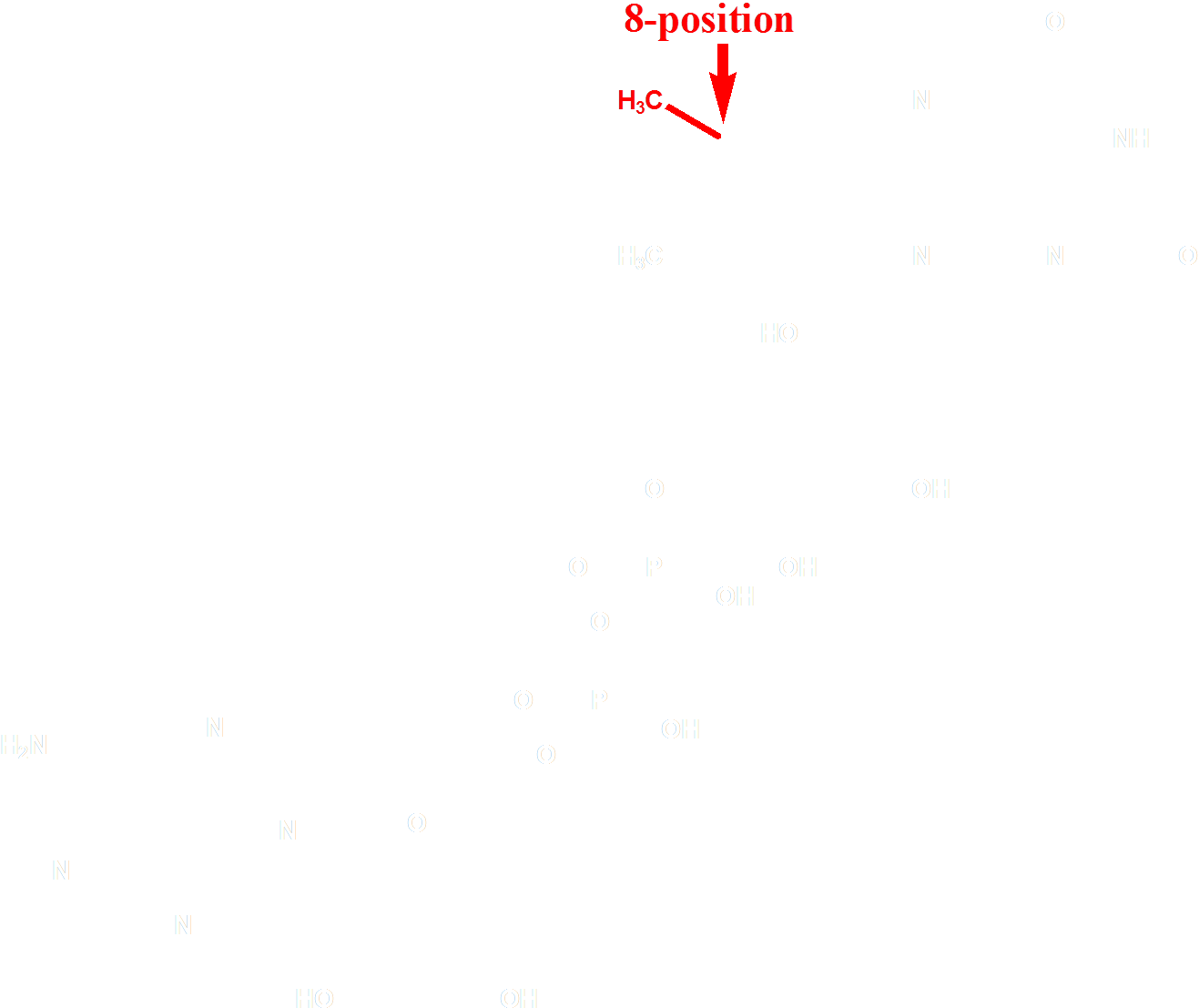
Figure 3 Molecular structure of FAD
Design
The choice of the FAD analogue should have the following features: a red-shifted absorption spectrum and a substitution at 8 position. After selecting from of a pool of over 500 chemicals listed by similarity search on SciFinder, we identified 8-tetrahydropyrroriboflavin (Fig. 4) as a promising candidate for incorporation, which has a red shift of absorption at 510nm.
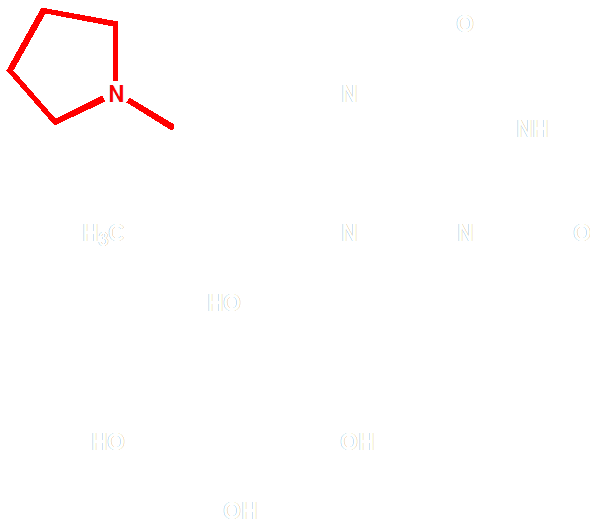
Figure 4 Molecular Structure of 8-tetrahydropyrrole riboflavin
On the other hand, in order to attain bio-orthoganality and to efficiently accommodate the FAD analogue, necessary mutations should be done with the pocket of VVD. Molecular docking results (Fig. 5) indicate that this analogue interacts with the protein at four specific sites(residues marked in blue). These side chains serve as spatial hindrances in that they are within a distance of 3 angstrom (dash lines). And mutations of these residues will hopefully create enough space for the 8-substituted FAD analogue, in this case, a tetrahydropyrrole is at the 8 position.
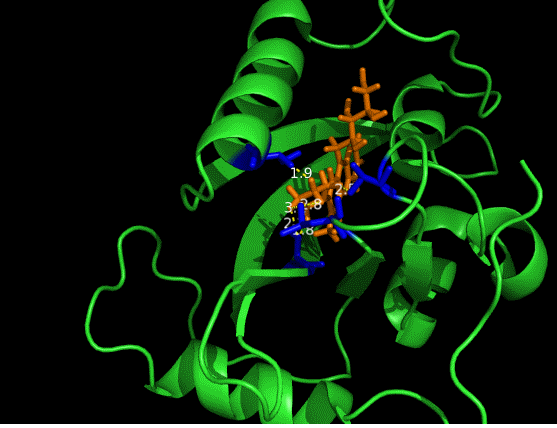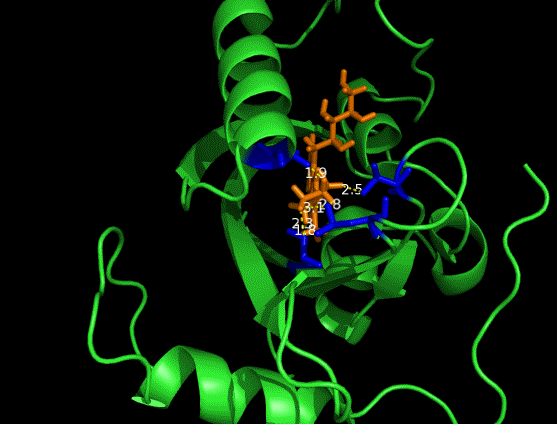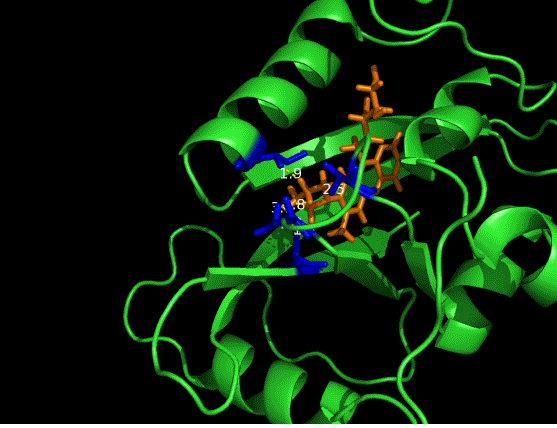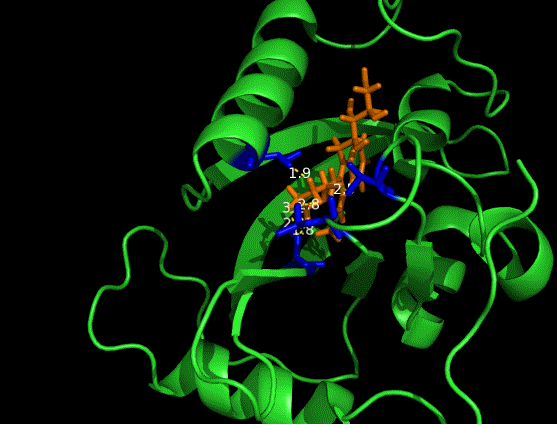
Figure 5 Spatial interactions between FAD analogue and the pocket of VVD
Perspectives
A newly developed, dual channel photosensing system based on LexA-VVD will greatly broaden the territory of optogentics. And a red-shifted luminesensor could be used in deep tissue phototherapy, non-invasive light control of neuron and many other fields. What’s more, the concept used in this design could be instructive for future endeavors in developing a spectroscopically different photosensor.
 "
"














