Team:Tuebingen/NotebookReports
From 2012.igem.org
Jakobmatthes (Talk | contribs) (→Weekly Reports) |
Jakobmatthes (Talk | contribs) (→Weekly Reports) |
||
| Line 5: | Line 5: | ||
These illustrations give a general overview to our approach of lab work. | These illustrations give a general overview to our approach of lab work. | ||
{| | {| | ||
| - | | [[File:Tue-map-parts.png| | + | | [[File:Tue-map-parts.png|200px|thumb|preparation of parts]] |
| - | | [[File:Tue-map-vector.png| | + | | [[File:Tue-map-vector.png|200px|thumb|preparation of vectors]] |
|- | |- | ||
| - | | [[File:Tue-map-assembly.png| | + | | [[File:Tue-map-assembly.png|200px|thumb|assembly]] |
| - | | [[File:Tue-map-shipping.png| | + | | [[File:Tue-map-shipping.png|200px|thumb|parts for shipping]] |
|} | |} | ||
== Parts, Plasmids and Constructs == | == Parts, Plasmids and Constructs == | ||
Revision as of 07:39, 23 September 2012
Weekly Reports
Procedure
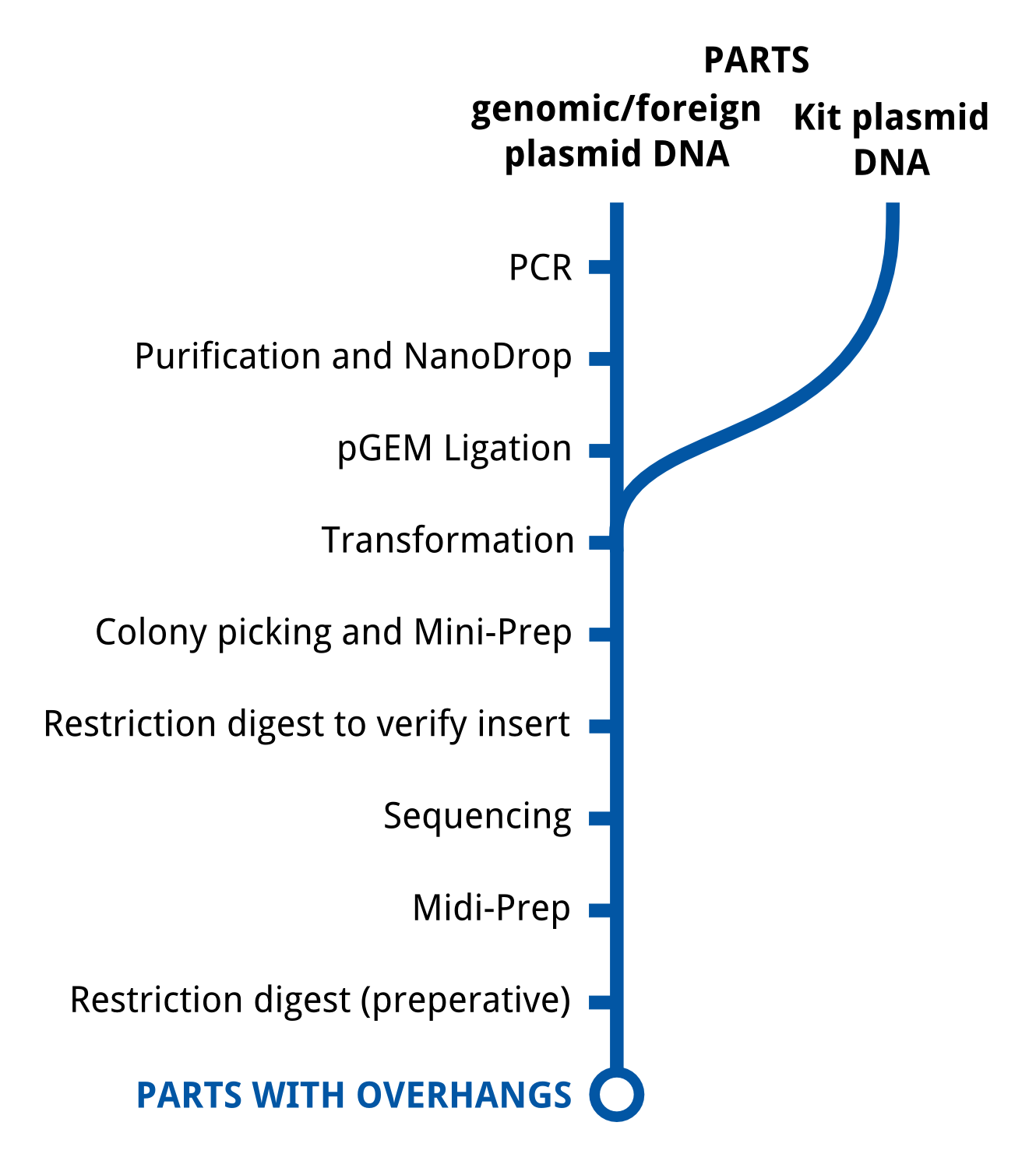
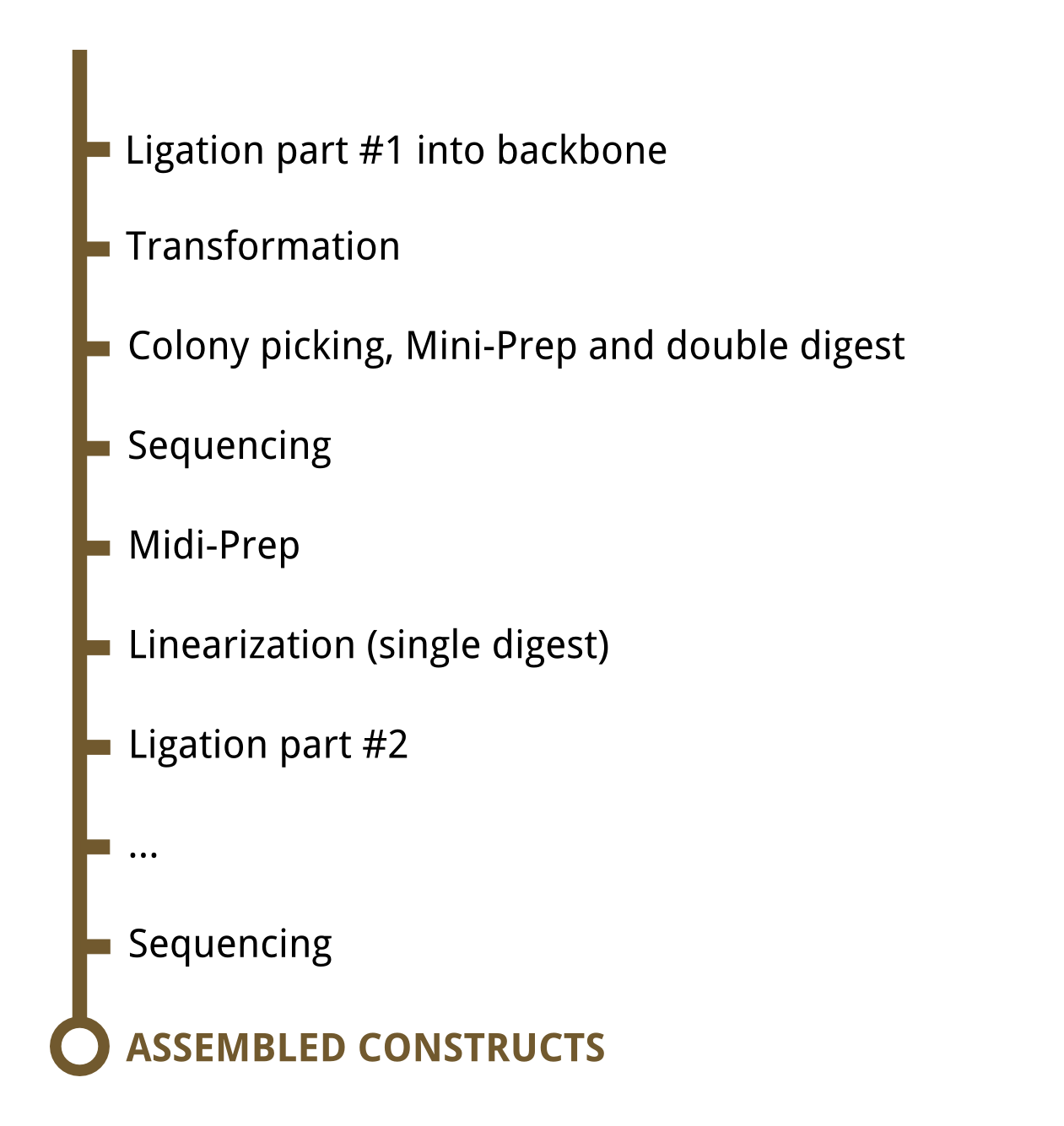
These illustrations give a general overview to our approach of lab work.
Parts, Plasmids and Constructs
To understand all referenced parts and their enumeration, here is a full listing:
| Part # | Part name | Registry Part |
|---|---|---|
| 1 | lacZ | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K950005 BBa_K950005] |
| 2 | luciferase | [http://partsregistry.org/wiki/index.php?title=BBa_K950004 BBa_K950004] |
| 3 | Padh1 | [http://partsregistry.org/wiki/index.php/Part:BBa_K165015 BBa_K165015] |
| 4 | Psuc2 | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K950003 BBa_K950003] |
| 5 | Pfet3 | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K950000 BBa_K950000] |
| 6 | Panb1 | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K950002 BBa_K950002] |
| 7 | Tadh1 | |
| 8 | rox1 | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K950001 BBa_K950001] |
| 9 | mPR Danio rerio | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K950006 BBa_K950006] |
| 10 | mig1 | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K950009 BBa_K950009] |
| 11 | mPR Xenopus laevis | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K950007 BBa_K950007] |
| pRS313 vector | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K950008 BBa_K950008] | |
| pRS315 vector | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K950010 BBa_K950010] | |
| pRS316 vector | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K950011 BBa_K950011] |
For the full information of these parts in the Parts Registry, refer to Submitted Parts.
Week 1 (7/9 - 7/15)
Thursday the 12th of July was the first day in our laboratory.
1. At first different substances, for example LB, SOB and TAE buffer 50x, which would be necessary for the further practice, were prepared.
2. To determine the optimal annealing temperature, a gradient PCR for the parts 1-8 was performed. Doing a gel-electrophoresis the PCR results were tested.
3. Preparing chemocompetent E. coli TOP 10 cells after Inoue protocol.
Week 2 (7/16 - 7/22)
1. A successful PCR for the Parts 1-7 with the optimal annealing temperature was performed. It was controlled by a gel-electrophoresis.
The Parts 3-7 were cleaned with a PCR-DNA-Purification-Kit. After that the concentration of the purified parts was measured with NanoDrop.
2. To test the competence of the chemocompetent E. coli TOP 10 cells a transformation with pRS313 and a negative control was done. Due to the fact that the competent cells didn't work, new chemocompetent E. coli TOP 10 cells were prepared. The transformation of pRS313, pRS315 and pRS316 in these competent cells was successful.
Week 3 (7/23 - 7/29)
1. The first [[1]] of the parts 1-8 in pGEM with following transformation in the competent E. coli TOP10 was done. But unfortunately only a few colonies grew on the inoculated agar-plates, which were incubated over night at 37°C.
The restriction digest was performed on the extracted plasmids of the grown colonies to control the ligation of the insert. The following gel electrophoresis showed that the ligation was not successful, because only bands of 3000bp for the pGEM vector was visible, but no bands for the insert.
2. A new PCR of the parts 1 and 8 was executed. Using a PCR-DNA-Purification-Kit the PCR-product of part 8 was purified. The PCR-product of part 1 was purified with a preparative gel. The concentration of the final products was measured with NanoDrop.
Week 4 (7/30 - 8/05)
1. The shipment with the synthesized parts (mPR of Danio rerio and mig1) arrived.
2. A transformation of the parts mPR Danio rerio and mig1 was performed using the competent E. coli TOP10 cells. Another transformation of the backbone plasmids pRS313, pRS315 and pRS316 was executed. Both were successful.
The first attempt to isolate the plasmids was through usage of a plasmid preparation kit, but this try failed. Therefore the plasmid isolation was successfully repeated using alkaline lysis.
3. A new PCR of the parts 1-8 with Taq/Pfu polymerase was performed applicating new yeast DNA. As an effect of the frequent freezing and defrosting the old yeast DNA was probably destroyed. Therefore some earlier PCRs did not work.
Week 5 (8/06 - 8/12)
1. A small restriction digest of the shuttle vectors pRS313, pRS315 and pRS316 was performed with XbaI and SpeI in order to examine the capability to linearize with the right overhangs for a ligation to take place later. The restriction digest was executed with the parts mig1 and mPR of Danio rerio, too. Due to unclean plasmids and DNA (perhaps to much salt) this step had to be repeated several times, because the restriction digests were incomplete.
Therefore the plasmids (pRS313, pRS315, pRS316 and the parts mig1, mPR Danio rerio) were purified again with a Midi Prep DNA purification kit. Now the restriction digest was executed completely. A mini plasmid preparation was performed afterwards to purify DNA in order to prepare the DNA for ligation.
2. A new PCR of the parts 1-8 was executed using Herculase in order to obtain a higher amount of PCR product. The polymerase Herculase was used due to its precision and productivity. Indeed the result of the PCR was better than with the Pfu/Taq polymerase.
A preparative gel for PCR products 3, 4, 5, 6, 7, 8 (from PCR with Herculase) delivered new template DNA for another PCR with Taq/Pfu Polymerase.
Week 6 (8/13 - 8/19)
1. The first successful ligation and transformation into pGEM vector of part 4 in E. coli TOP10 was executed. A lot of colonies grew on the agar-plate. After a restriction digest with XbaI and SpeI and the control with a gel electrophoresis the right band of 711bp was visible. The sequencing of the DNA confirmed that part 4 has the expected nucleotide sequence. A Midi-Prep, restriction digest and preparative gel electrophoresis followed in order to prepare them for later ligation into pRS vectors.
Ligation of part 3, 6, 7 and 8 in pGEM vector was performed. Reaction took place over night at 4 °C. The transformation of these parts was executed into E. coli TOP10. After growth over night, a mini plasmid preparation was performed. After a colony-PCR with parts 3, 6, 7, 8 did not work, we had to go back to the restriction digest for insert controllin. Positive samples were prepared for sequencing. The parts 3 and 8 were sequenced successfully and yielded a good sequence. The ligation of parts 6 and 7 failed, so we decided to skip part 6, because we may use Psuc2 as an alternative promotor for luciferase.
2. We received the synthesized receptor of Xenopus laevis. It was successfully transformed in E. coli TOP10 and purified with a Midi-Prep.
Week 7 (8/20 - 8/26)
1. The PCR of the parts 1 and 2 with Herculase polymerase was executed. The PCR products were checked with an analytical gel afterwards. The PCR of part 1 failed again, so we decided to reject part 1 and continue working only with luciferase (part 2), because we only need one reporter gene.
2. Since we ran out of luciferase plasmid DNA, we decided to transformation the remaining DNA of luciferase into E. coli TOP10. The transformation was successful. A Midi-Prep yielded new plasmid DNA.
3. The receptors (mPR Danio rerio, mPR Xenopus laevis) and mig1 were initially ligated into pRS vectors and transformed into E. coli TOP10. But no colonies grew on the agar-plates.
Week 8 (8/27 - 9/02)
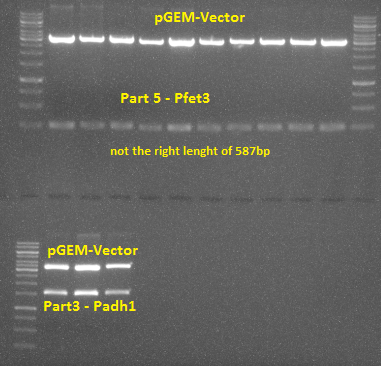
1. Part 5 was ligated into pGEM vector and transformed into E. coli TOP10 afterwards. After performing a restriction digest it was obvious that the insert did not have the correct length and therefore has to be discarded.
2. Second ligation of mPR Danio rerio, mPR Xenopus laevis and mig1 into pRS vectors and transformation into E. coli TOP10 was executed. Some colonies grew on the agar-plates. Therefore a mini-prep and a restriction digest with XbaI and SpeI with a following gel electrophoresis was conducted. But there was only ligation of the insert into pGEM, not into pRS. The hypothesis was that the pGEM constructs were contamination.
3. Ligation of the parts 3 and 4 in pRS vectors with following transformation into E. coli TOP10 was performed. But it was not successful.
 "
"