Team:Cambridge/Lab book/Week 7
From 2012.igem.org
(Difference between revisions)
(→Sunday (12/08/12)) |
(→Sunday (12/08/12)) |
||
| Line 89: | Line 89: | ||
===Sunday (12/08/12)=== | ===Sunday (12/08/12)=== | ||
| + | |||
| + | '''[[Team:Cambridge/Protocols/Gibsonassembly|Assembly of sfGFP construct from known functional DNA fragments]]''' | ||
| + | |||
| + | ---- | ||
| + | |||
| + | *Fragments provided by '''who?''' assembled with Gibson. | ||
'''[[Team:Cambridge/Protocols/TransformationofE.coli|Transformation of e.coli with fluorescent construct and sfGFP positive control]]''' | '''[[Team:Cambridge/Protocols/TransformationofE.coli|Transformation of e.coli with fluorescent construct and sfGFP positive control]]''' | ||
Revision as of 09:44, 14 August 2012
| Week: | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|
Contents |
Monday (06/08/12)
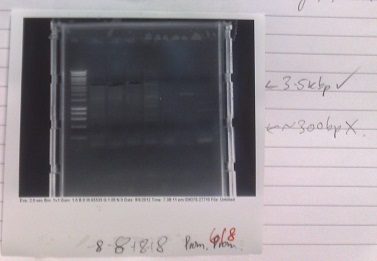
PCR of Magnesium riboswitch vector fragment B and Magnesium promoter
- Normal PCR settings used, annealing temperature 57 °C, elongation step 90s long.
- Lane 5 accidentally loaded with a DNA ladder instead of loading dye.
- Expected fragment sizes:
- Lane 2-5: 3kbp
- Lane 6-7: 300bp
- After electrophoresis, found vector products had, for the most part, worked. Promoter elements were not amplified - no band of the expected size was observed.
- Positive control also failed, although this has ceased to work for several days, potentially due to DNA degradation.
Tuesday (07/08/12)
Production of electrocompetent e.coli
Gibson assembly of magnesium riboswitch and fluorescent construct
- NAD+ added to isothermal buffer*5 mix
- Gel slices from yesterday (of vector fragment B) purified.
- DNA added as follows:
- Without 8 codon substitution:
- Reaction 1: Tube 2 (07/08/12) (fragment B), Tube 1 (05/08/12) (fragment A), Tube 28 (29/08/12) (riboswitch DNA).
- Reaction 2: Tube 3 (07/08/12) (fragment B), Tube 2 (05/08/12) (fragment A), Tube 29 (29/08/12) (riboswitch DNA).
- With 8 codon substitution:
- Reaction 3: Tube 4 (07/08/12) (fragment B), Tube 1 (05/08/12) (fragment A), Tube 2 (18/07/12) (riboswitch DNA).
Wednesday (08/08/12)
Electrical transformation of competent e.coli with Gibson products
- Gibson constructs from yesterday (fluorescent and magnesium riboswitch) transformed into e.coli produced yesterday.
- Cells plated out onto 50 μg/ml ampicillin plates. Put in incubator overnight.
Thursday (09/08/12)
Making chemically competent e.coli
- 1l SOB made up.
- Split mOrange vector amplification attempted again.
Friday (10/08/12)
Saturday (11/08/12)
Sunday (12/08/12)
Assembly of sfGFP construct from known functional DNA fragments
- Fragments provided by who? assembled with Gibson.
Transformation of e.coli with fluorescent construct and sfGFP positive control
- Chemically competent e.coli cells transformed with fluorescent construct Gibson product from 07/08/12.
- E.coli cells also transformed with sfGFP Gibson product made earlier today in triplicate.
- All transformants plated out on 100 μg/ml ampicillin.
- sfGFP fragments have produced successful Gibson products in the past, so this will act as our positive control. If cells grow with the plasmid and fluoresce properly, we will know the master mix works. Otherwise, we will remake the master mix.
 "
"