Team:Austin Texas/Spinach reporter
From 2012.igem.org
(→Issues with results and mCherry Not Working) |
|||
| Line 71: | Line 71: | ||
Given the difficulty with quantifying the translational strength due to poor mCherry signal, it was decided that focus would be put on characterizing the Spinach aptamer + stabilizing tRNA scaffold by itself. | Given the difficulty with quantifying the translational strength due to poor mCherry signal, it was decided that focus would be put on characterizing the Spinach aptamer + stabilizing tRNA scaffold by itself. | ||
| + | ===Spinach Aptamer=== | ||
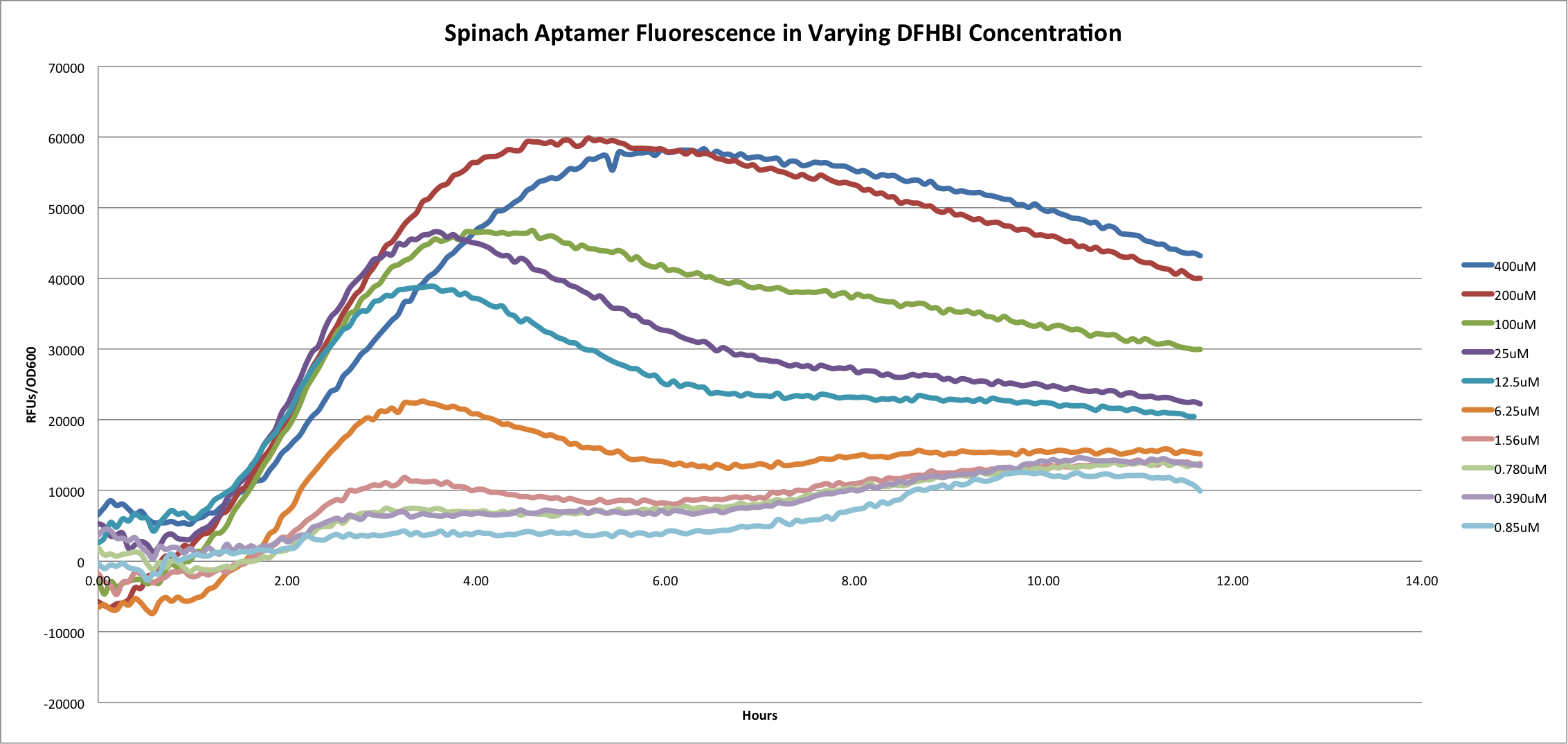
[[File:Spa Over Hours.png|center|800px]] | [[File:Spa Over Hours.png|center|800px]] | ||
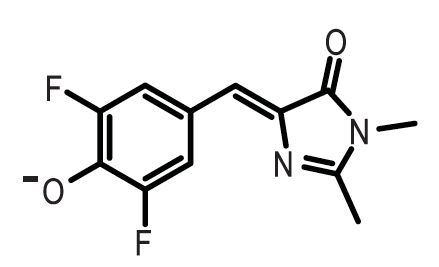
This graph shows the fluorescence of the Spinach Aptamer [http://partsregistry.org/Part:BBa_K734002 BBa_K734002] over time in a dilution series of DFHBI concentrations. DFHBI, 3,5-difluoro-4-hydroxybenzylidene imidazolinone (DFHBI), shown below, is a synthetic fluorophore designed in the Jaffrey Lab at Cornell University. When spinach mRNA binds to DFHBI, it exhibits green fluorescence, which we utilized as a method to measure promoter strength. Based on the curve of 400uM DFHBI, it appears that cell growth is hindered at higher concentrations of this synthetic fluorophore, leading us to utilize 200uM DFHBI as the optimal concentration for maximizing OD600 and RFU of the Spinach aptamer. | This graph shows the fluorescence of the Spinach Aptamer [http://partsregistry.org/Part:BBa_K734002 BBa_K734002] over time in a dilution series of DFHBI concentrations. DFHBI, 3,5-difluoro-4-hydroxybenzylidene imidazolinone (DFHBI), shown below, is a synthetic fluorophore designed in the Jaffrey Lab at Cornell University. When spinach mRNA binds to DFHBI, it exhibits green fluorescence, which we utilized as a method to measure promoter strength. Based on the curve of 400uM DFHBI, it appears that cell growth is hindered at higher concentrations of this synthetic fluorophore, leading us to utilize 200uM DFHBI as the optimal concentration for maximizing OD600 and RFU of the Spinach aptamer. | ||
| + | |||
| + | Since this project utilized one promoter, monitoring Spinach aptamer fluorescence over time is shown to be a reliable way to characterize promoters. Future directions for this project include creating a library of constitutive T7 promoters, as well as looking into secondary structure strength by inserting degenerate bases into the Spinach aptamer structure and monitoring fluorescence. | ||
[[File:156.jpeg|lright|200px|]] | [[File:156.jpeg|lright|200px|]] | ||
Revision as of 23:13, 3 October 2012

Contents |
Spinach/mCherry Dual flourescence Reporter
Background
In an effort to improve both efficiency, ease, and quality of promoter and RBS strength measurements, we focused on developing a dual fluorescence reporter for simultaneous monitoring both transcription and translation. To measure both processes separately, two fluorescent reporters, the Spinach aptamer and mCherry red fluorescent protein, were assembled into a single construct. The Spinach-mCherry dual reporter is a unique concept; Spinach is a short RNA aptamer that binds to its ligand, DFHBI, and allows it to emit green fluorescence similar to GFP. This gives insight into the direct production of the mCherry-encoding mRNA without the need to wait for protein folding and maturation of the fluorophore. This technique attempted to expand upon current efforts to measure promoter strength relative to a reference standard used by the iGEM community.
Design
The reporter was designed in two ways: constructs with the mCherry gene 5' of the spinach aptamer, and with the mCherry gene 3' of the spinach aptamer. The construct was assembled in a pET plasmid, with the spinach aptamer containing a small, stabilzing tRNA scaffold on both sides of aptamer. (original Spinach pET plasmid was obtained from Xi Chen of the Ellington Lab, UT Austin) The plasmid containing mCherry, pAAV-miniCMV-mCherry was obtained from Addgene (Addgene plasmid 27970, Church lab Harvard University.) Through the use of gibson assembly, the the mCherry fluorescent protein gene was inserted into the spinach pET plasmid, at both 5' and 3' locations of the spinach aptamer. Little troubleshooting was needed as the gibson worked after one attempt. However, many errors were encountered when using various fluorescent plate readers, often wasting whole trials due to machine detection error. (more detail?) The promoter that would be tested for Spinach fluorescence was the T7 Promoter: TAATACGACTCACTATAGGGT, resembles closely [http://partsregistry.org/wiki/index.php?title=Part:BBa_J64997 Bba_J64997] constitutive T7 promoter.
5' Spinach/mCherry Dual Reporter
This construct contains the mCherry gene 5' of the Spinach aptamer, we assumed this construct would be more effective at showing translational strength because of the possibility of the Spinach Aptamer secondary structure knocking off the ribosome during translation.
3' Spinach/mCherry Dual Reporter
This version of the construct has mCherry 3' of the Spinach aptamer, we had expected this construct would not be as efficient as the 5' construct.
Results
Protocol
The protocol for using the dual fluorescent reporter was performed as follows:
- Grow culture of construct (both 5' and 3') overnight in BL21 AI or BL21 DE3 (BL21 contains gene coding for T7 RNAP under control of LacUV5)
- Dilute on the following day and grow until mid-log phase
- Induce BL21 cells (DE3 or AI) with either 1mM IPTG or 1mM IPTG and 0.2% arabinose, respectively.
- Add 200uL aliquots of construct and control cells in a 96-well plate
- The fluorphore for spinach fluorescence, DFHBI, was added either into the well plate if performing a dilution series, or directly into the culture of cells
- Dilution series of DFHBI, revealed 100-200uM DFHBI being the ideal concentration in culture for max RFU values and minimal cell death
- Analyze graphs of Spinach Fluorescence (RFU/ABS) vs Time
- RFU/ABS calculated by RFUtest / OD600test - RFUcontrol / OD600control = RFU/ABS
Data
The following graphs have been created from measuring Spinach Aptamer fluorescence at 501nm over time in a Tecan [http://www.tecan.com/platform/apps/product/index.asp?MenuID=1812&ID=1916&Menu=1&Item=21.2.10.1 F 200] plate reader.
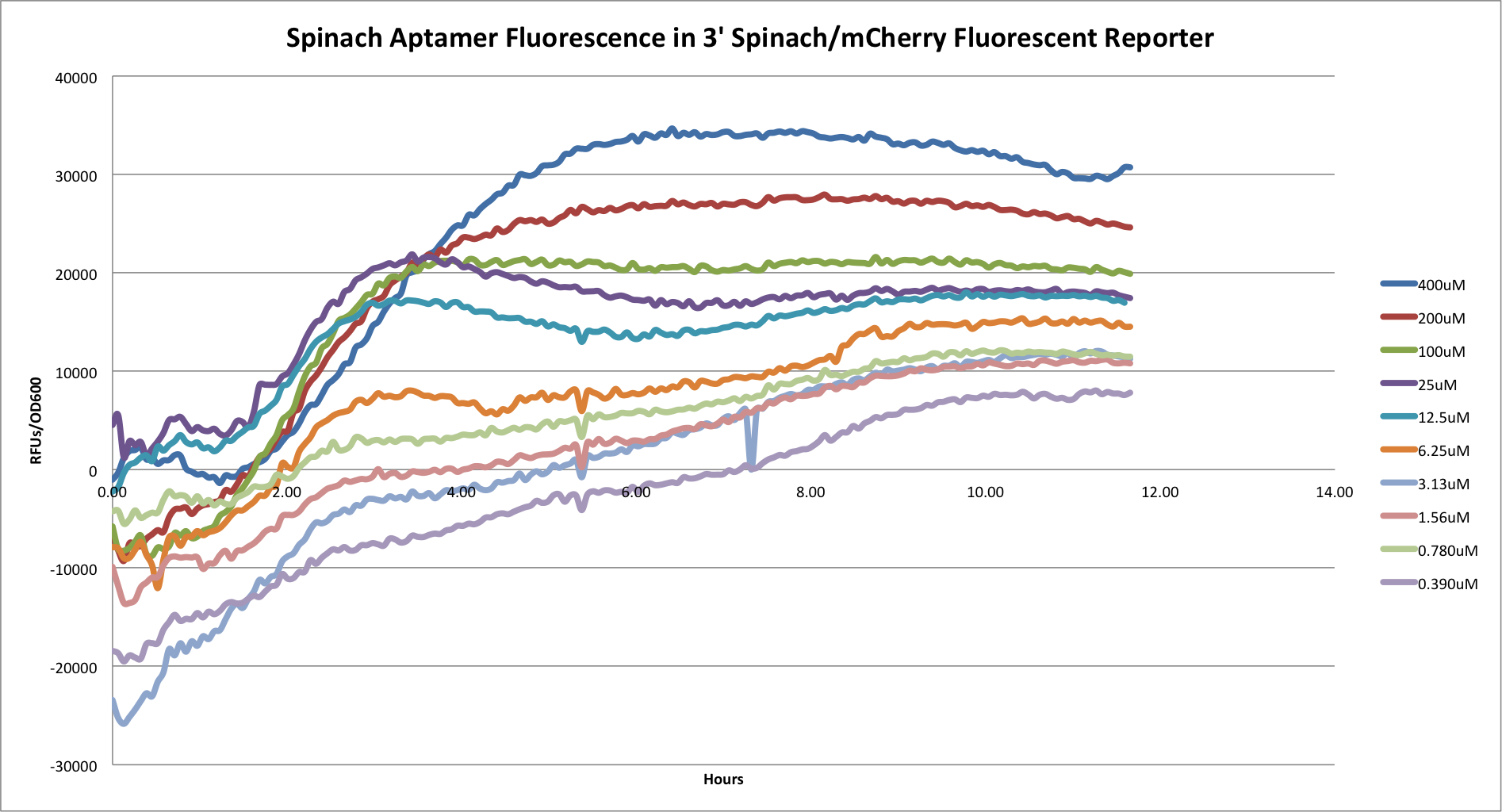
- 3' Spinach/mCherry Reporter
This graph shows the Spinach aptamer fluorescence of the 3' construct over time. This construct functioned very similarly to the 5' construct (picture below). As well, neither construct functioned well in terms of mCherry fluorescence.
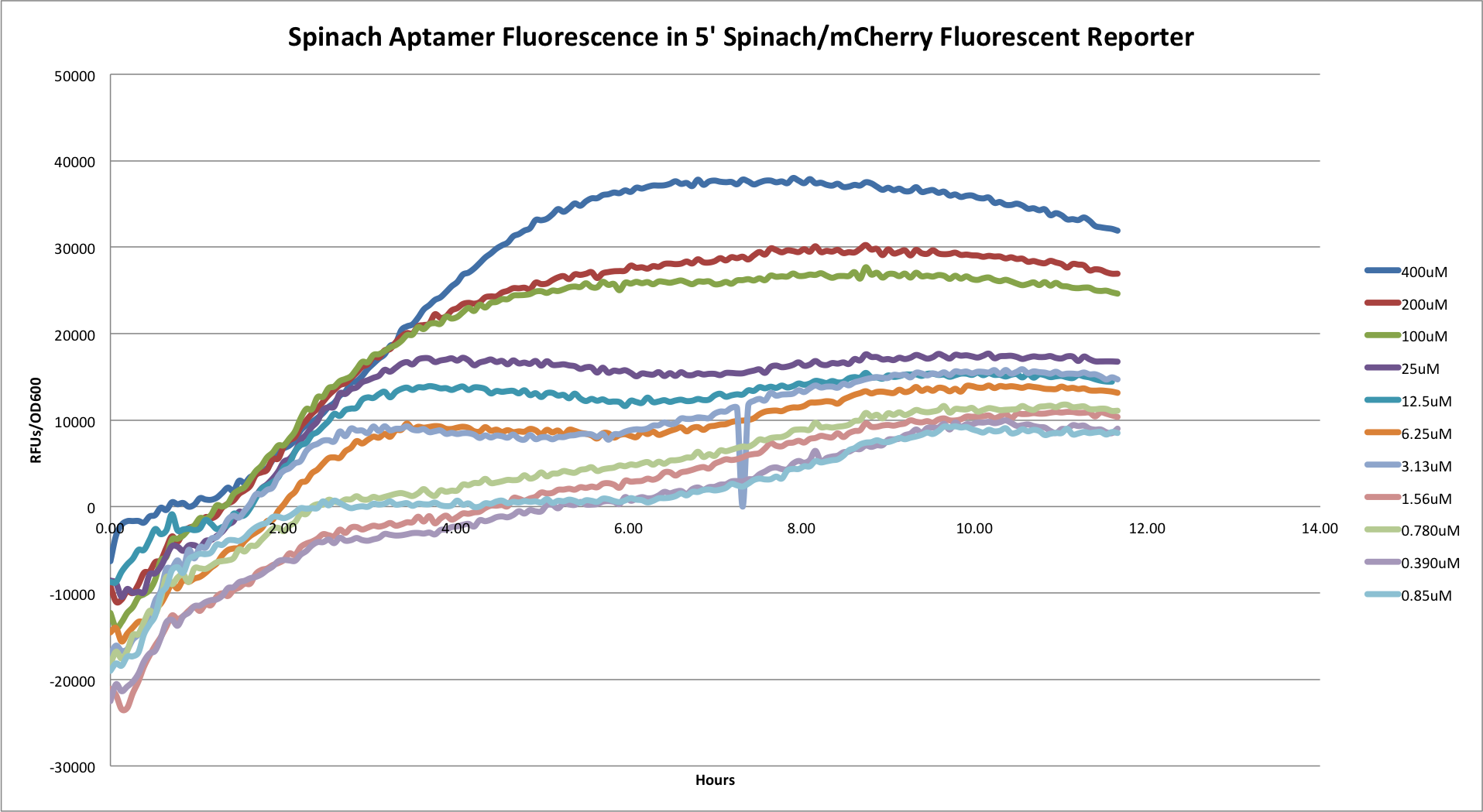
- 5' Spinach/mCherry Reporter
Graph showing the Spinach aptamer fluoresence in the 5' construct.
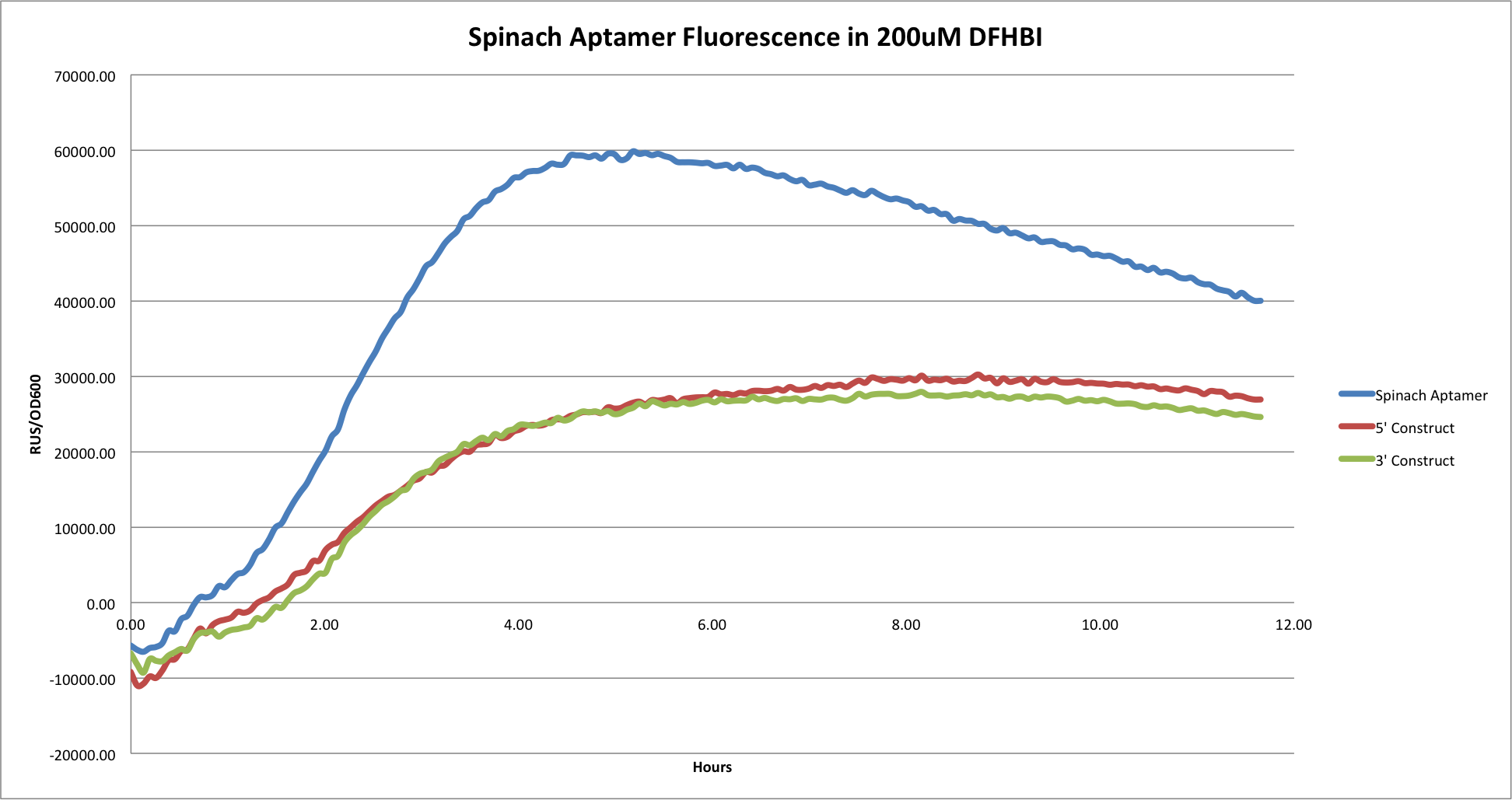
- Comparison of Spinach Aptamer by itself, 5' construct, and 3' construct in 200uM DFHBI.
This graph shows the comparison of the Spinach aptamer by itself, the 3', and 5' constructs. As depicted, the larger constructs that contained the ~800bp mCherry gene had overall less fluorescence and over all lower OD600 values. It is likely that attaching a large gene such as this to the Spinach tRNA results in overall less tRNA and less expression of Spinach aptamer fluorescence.
Issues with results and mCherry Not Working
After considerable time using the Spinach+mCherry dual fluorescence reporter it became clear that mCherry was not developing in the plate reader during fluorescence experiments, but this was not immediately obvious. Originally, the plate reader being used for this project was showing no fluorescence change in either the wavelengths of spinach or mcherry (Spinach- ex: 469nm em: 501nm, mCherry- ex:587 em: 610nm), the assumption was made that the construct was not performing as intended. It wasn't until trying multiple other flurescence plate readers that we stumbled upon a Tecan F200 plate reader in the Zhang lab (UT Austin) that was able to detect the spinach fluorescence. Once the plate reader issue had been realized, it became obvious that the mCherry fluorescent protein was not able to develop fully during the plate reader experiments. This can likely be contributed to the low oxygen conditions that existed in the machine during fluorescence trials, as well other issues, including the demanding qualities of the construct itself.
Given the difficulty with quantifying the translational strength due to poor mCherry signal, it was decided that focus would be put on characterizing the Spinach aptamer + stabilizing tRNA scaffold by itself.
Spinach Aptamer
This graph shows the fluorescence of the Spinach Aptamer [http://partsregistry.org/Part:BBa_K734002 BBa_K734002] over time in a dilution series of DFHBI concentrations. DFHBI, 3,5-difluoro-4-hydroxybenzylidene imidazolinone (DFHBI), shown below, is a synthetic fluorophore designed in the Jaffrey Lab at Cornell University. When spinach mRNA binds to DFHBI, it exhibits green fluorescence, which we utilized as a method to measure promoter strength. Based on the curve of 400uM DFHBI, it appears that cell growth is hindered at higher concentrations of this synthetic fluorophore, leading us to utilize 200uM DFHBI as the optimal concentration for maximizing OD600 and RFU of the Spinach aptamer.
Since this project utilized one promoter, monitoring Spinach aptamer fluorescence over time is shown to be a reliable way to characterize promoters. Future directions for this project include creating a library of constitutive T7 promoters, as well as looking into secondary structure strength by inserting degenerate bases into the Spinach aptamer structure and monitoring fluorescence.
- 3,5-difluoro-4-hydroxybenzylidene imidazolinone (DFHBI)
 "
"