Team:Stanford-Brown/HellCell/pH
From 2012.igem.org
(→pH At a glance Extremophile: Escherichia coli Proteins of interest: serine deaminase (serine catabolism) Consensus: Helpful) |
(→pH At a glance Extremophile: Escherichia coli Proteins of interest: serine deaminase (serine catabolism) Consensus: Helpful) |
||
| Line 26: | Line 26: | ||
~analysis of data~ | ~analysis of data~ | ||
| + | |||
| + | '''Why not Acid??''' | ||
| + | |||
| + | So, why didn’t we include acid, you ask? We did not forget it, we promise! Like alkaliphiles, acidophiles use ATP synthases to maintain their internal pH at hospitable levels. However, as already mentioned, these synthases are large and difficult to control (Foster 2004). Acidophiles also have membranes made up of tetraether lipids instead of ester linkages, which is difficult to engineer (Baker-Austin and Dopson). ''Helicobacter pylori'', a bacterium that inhabits the stomach and causes ulcers, thrive in the corrosively acidic environment of the stomach (pH 2-5). Central to this capacity is the breakdown of urea into ammonia and carbon dioxide, catalyzed by the enzyme urease (Labigne, Cussac, and Courcoux 1991). It was found, however, that several genes are necessary for expression of urease and urease-like phenotypes in ''E. coli'', making it difficult to BioBrick (Cussac, Ferrero, Labigne 1992). Amino acid degradation is another mechanism common to both alkaliphiles and acidophiles—both glutamate and arginine can be broken down to produce buffers (Foster 2004). Arginine, however, is not present in high concentrations in LB broth, and the three genes involved in glutamate catabolism (gadA, gadB, and gadC) and several BioBrick restriction enzyme cut sites which we did not have time to engineer around. | ||
| + | |||
Sources: Padan, E., Bibi, E., Ito, M., Krulwich, T.A. (2005). Alkaline pH Homeostasis in Bacteria: New Insights. ''Biochim Biophys Acta, 1717''(2): 67-88. | Sources: Padan, E., Bibi, E., Ito, M., Krulwich, T.A. (2005). Alkaline pH Homeostasis in Bacteria: New Insights. ''Biochim Biophys Acta, 1717''(2): 67-88. | ||
| + | |||
| + | Foster, J. W. (2004). ''Escherichia Coli'' acid resistance: Tales of an amateur acidophile. ''Nature Rev. Microbiol., 2'', 898-907. | ||
| + | |||
| + | Labigne, A., Cussac, V., Courcoux, P. (1991). Shuttle cloning and nucleotide sequences of ''Helicobacter pylori'' genes responsible for urease activity. ''J. Bacteriol., 173''(6): 1920-1931. | ||
| + | |||
| + | Cussac, V., Ferrero, R. L., Labigne, A. (1992). Expression of Helicobacter pylori urease genes in Escherichia coli grown under nitrogen-limiting conditions. ''J. Bacteriol., 174''(8): 2466-2473. | ||
| + | |||
| + | Baker-Austin, C. Dopson, M. (2007). Life in acid: pH homeostasis in acidophiles. ''Trends in Microbiology, 15''(4): 165-170. | ||
Revision as of 04:24, 2 October 2012
pH
At a glance
Extremophile: Escherichia coli
Proteins of interest: serine deaminase (serine catabolism)
Consensus: Helpful
Alkaliphiles like marine bacteria (pH 8.2) and pyloric duct flora (pH ~10 or higher) rely largely on transmembrane transporter proteins to regulate the pH within the cytoplasm and thrive (Padan, Bibi, Ito, and Krulwich 2005). Most notable of these transporters are ATP synthase and cation/proton antiporters. Both of these use some type of energy to power the transport of H+ ions across the membrane: in the former, the energy released in the dephosphorylation of ATP is coupled with the transport; in the latter, an electrochemical gradient is exploited (Padan, et al. 2005). All the genes required for these transmembrane proteins total ~10 kb in length, however, which is difficult to manage in BioBrick form. Additionally, these proteins are optimized for the membranes of alkaliphilic bacteria which have a different lipid composition than E. coli, making their behavior tricky to control in a planned fashion (Padan, et al. 2005).
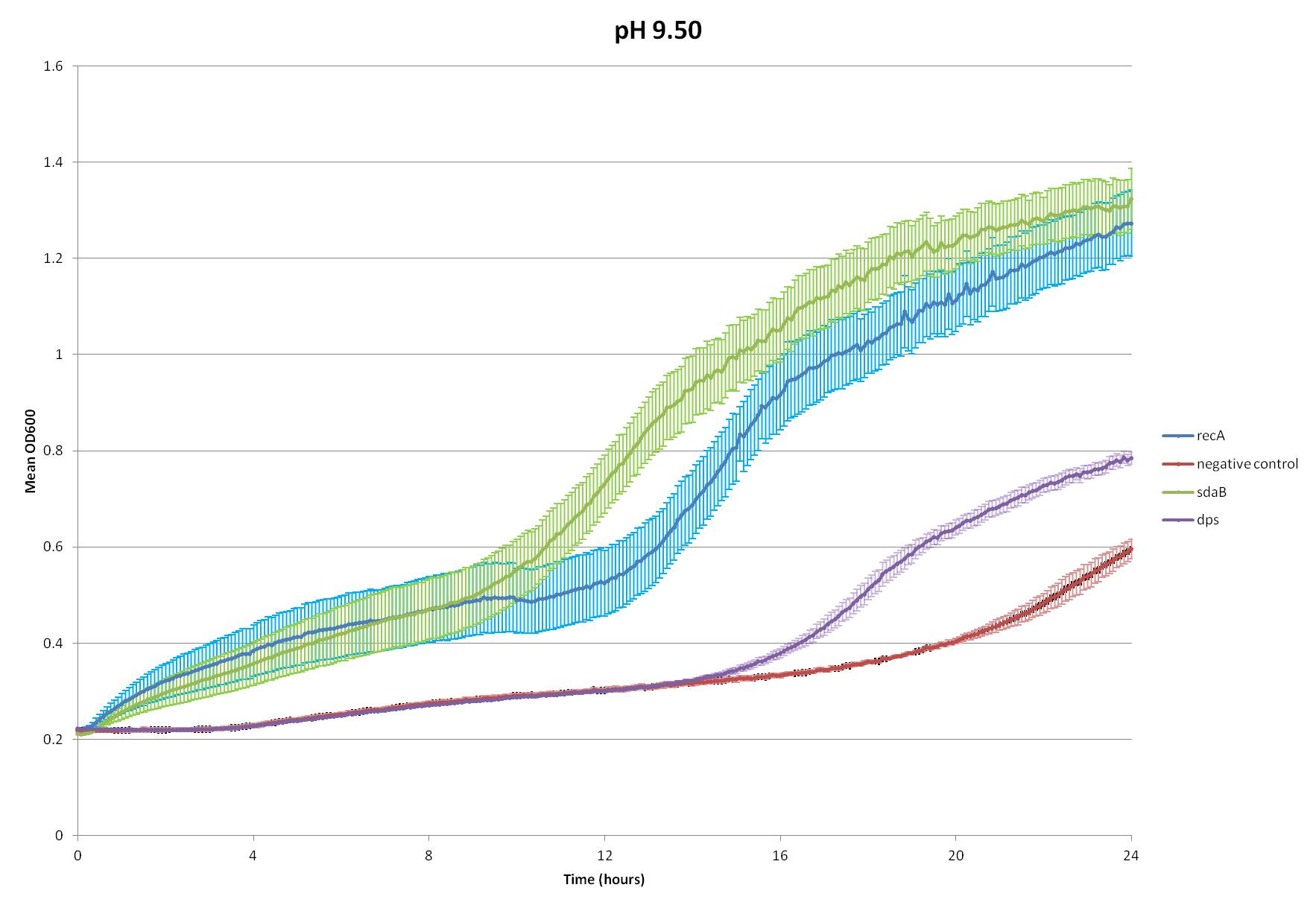
What the Hell Cell squad found intriguing, however, is that it has been found that E. coli increases its catabolism of amino acids when exposed to high pHs, most researched of which are tryptophan and serine (Padan et al.). This process creates buffers in the cytoplasm to help counter the effects of the high pH. Since the heightened catabolism of tryptophan would require the insertion of a tryptophan transporter, we decided to choose serine catabolism and isolated the gene for serine deaminase from E. coli.
We inserted this gene into the Test Plasmid, transformed E. coli with it, and assay its function. We also tested the effectiveness of two genes from our radiation assays, Dps and recA--see Radiation.
~stuff about protocol~
~analysis of data~
Why not Acid??
So, why didn’t we include acid, you ask? We did not forget it, we promise! Like alkaliphiles, acidophiles use ATP synthases to maintain their internal pH at hospitable levels. However, as already mentioned, these synthases are large and difficult to control (Foster 2004). Acidophiles also have membranes made up of tetraether lipids instead of ester linkages, which is difficult to engineer (Baker-Austin and Dopson). Helicobacter pylori, a bacterium that inhabits the stomach and causes ulcers, thrive in the corrosively acidic environment of the stomach (pH 2-5). Central to this capacity is the breakdown of urea into ammonia and carbon dioxide, catalyzed by the enzyme urease (Labigne, Cussac, and Courcoux 1991). It was found, however, that several genes are necessary for expression of urease and urease-like phenotypes in E. coli, making it difficult to BioBrick (Cussac, Ferrero, Labigne 1992). Amino acid degradation is another mechanism common to both alkaliphiles and acidophiles—both glutamate and arginine can be broken down to produce buffers (Foster 2004). Arginine, however, is not present in high concentrations in LB broth, and the three genes involved in glutamate catabolism (gadA, gadB, and gadC) and several BioBrick restriction enzyme cut sites which we did not have time to engineer around.
Sources: Padan, E., Bibi, E., Ito, M., Krulwich, T.A. (2005). Alkaline pH Homeostasis in Bacteria: New Insights. Biochim Biophys Acta, 1717(2): 67-88.
Foster, J. W. (2004). Escherichia Coli acid resistance: Tales of an amateur acidophile. Nature Rev. Microbiol., 2, 898-907.
Labigne, A., Cussac, V., Courcoux, P. (1991). Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J. Bacteriol., 173(6): 1920-1931.
Cussac, V., Ferrero, R. L., Labigne, A. (1992). Expression of Helicobacter pylori urease genes in Escherichia coli grown under nitrogen-limiting conditions. J. Bacteriol., 174(8): 2466-2473.
Baker-Austin, C. Dopson, M. (2007). Life in acid: pH homeostasis in acidophiles. Trends in Microbiology, 15(4): 165-170.
 "
"