Team:TU-Eindhoven/LEC/BioBricks
From 2012.igem.org
| Line 8: | Line 8: | ||
<h3>Characterization</h3> | <h3>Characterization</h3> | ||
<p>E. Coli strain BL21 transformed with Part BBa_K881000 and Part BBa_K881001 ligated into vector pET_28a were cultured in LB media containing 100 µg/ml kanamycin for ~4 hours at 37°C. The growth rate was followed by use of OD600 measurements (see figure on the right). Then the culture was induced at an OD600 of 0,82 absorption units with 100 µg/ml IPTG.</p> | <p>E. Coli strain BL21 transformed with Part BBa_K881000 and Part BBa_K881001 ligated into vector pET_28a were cultured in LB media containing 100 µg/ml kanamycin for ~4 hours at 37°C. The growth rate was followed by use of OD600 measurements (see figure on the right). Then the culture was induced at an OD600 of 0,82 absorption units with 100 µg/ml IPTG.</p> | ||
| - | |||
| - | |||
<h3>Harvesting and Purification</h3> | <h3>Harvesting and Purification</h3> | ||
<p>After induction for 15 hours, the cells were harvested and lysed with 5ml BugBuster containing 1µl/ml Benzonase. The supernatant of this suspension was put on a Ni-NTA column for purification and afterwards put on a PD10 column and washed with a TRIS buffer containing NaCl to remove all the calcium molecules. Then, an SDS-gel was prepared to check the purity of the GECO proteins (See figure below). | <p>After induction for 15 hours, the cells were harvested and lysed with 5ml BugBuster containing 1µl/ml Benzonase. The supernatant of this suspension was put on a Ni-NTA column for purification and afterwards put on a PD10 column and washed with a TRIS buffer containing NaCl to remove all the calcium molecules. Then, an SDS-gel was prepared to check the purity of the GECO proteins (See figure below). | ||
[[Image:SDS-gel.jpg|500px|left]]</p> | [[Image:SDS-gel.jpg|500px|left]]</p> | ||
| + | |||
| + | <h3>Calcium titration</h3> | ||
{{:Team:TU-Eindhoven/Templates/footer}} | {{:Team:TU-Eindhoven/Templates/footer}} | ||
Revision as of 19:55, 25 September 2012

The GECO’s, R-GECO (red), G-GECO (green) and B-GECO (blue) are the biobricks we will send to the registry. Those biobricks can be used in yeast and in E.coli cells. The protocol attached with the biobrick-library didn’t work for us. We used our own restriction and ligation protocols to develop the biobrick.
The permission we needed to add the GECO’s to registry library was not easy to get. You can read more about this struggle at the Considerations-page.
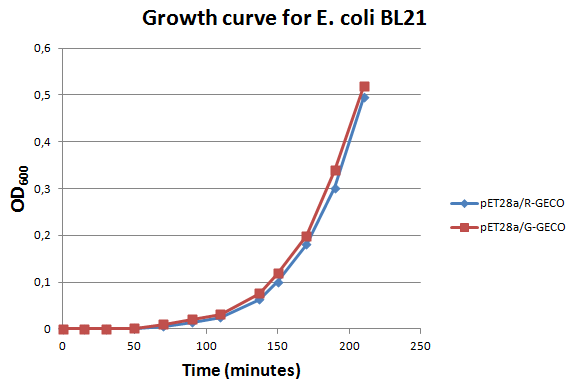
Characterization
E. Coli strain BL21 transformed with Part BBa_K881000 and Part BBa_K881001 ligated into vector pET_28a were cultured in LB media containing 100 µg/ml kanamycin for ~4 hours at 37°C. The growth rate was followed by use of OD600 measurements (see figure on the right). Then the culture was induced at an OD600 of 0,82 absorption units with 100 µg/ml IPTG.
Harvesting and Purification
After induction for 15 hours, the cells were harvested and lysed with 5ml BugBuster containing 1µl/ml Benzonase. The supernatant of this suspension was put on a Ni-NTA column for purification and afterwards put on a PD10 column and washed with a TRIS buffer containing NaCl to remove all the calcium molecules. Then, an SDS-gel was prepared to check the purity of the GECO proteins (See figure below).
Calcium titration
 "
"